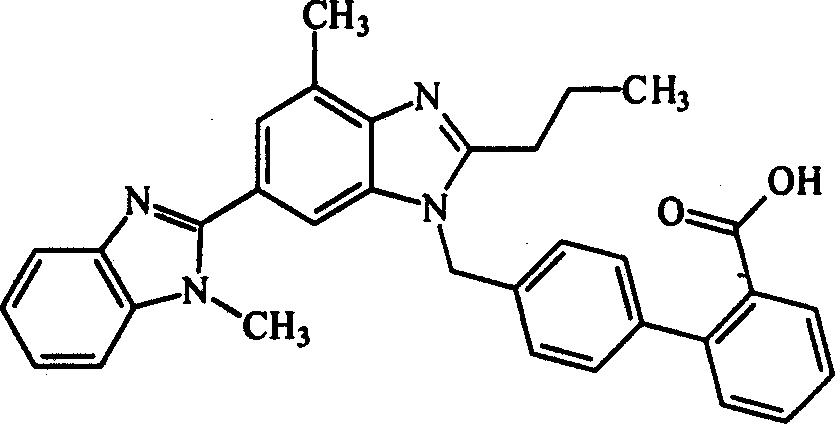
Tilmisartan salt and its prepn
A technology of telmisartan and sodium salt, which is applied in the field of telmisartan salt and its preparation, can solve the problems of low solubility, low dissolution rate and strong water absorption of telmisartan raw materials, and achieve high promotion and application prospects , High dissolution rate, easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
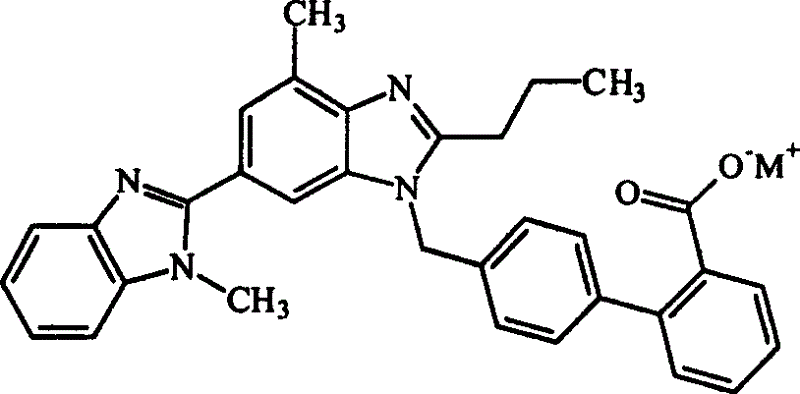
[0026] 4'-[(1,4'-dimethyl-2'-n-propyl[2,6'-di-1hydro-benzimidazol]-1'-yl)methyl]-[1,1' -Biphenyl]-2-carboxylic acid sodium salt
[0027] In a 250ml reaction flask, add 10g (0.0195mol) Telmisartan (0.0195mol), 0.75g (0.0189mol) NaOH and 100ml water, stir for 1 hour (30°C), filter, remove insoluble matter, concentrate to a small volume, add ethanol 30ml, concentrated, added 30ml of n-hexane to wash, poured off, added 30ml of ethanol, concentrated, repeated again, concentrated to dryness to obtain 9.9g of telmisartan sodium salt, yield 95.2%. Melting point: 223-225°C.
[0028] Elemental Analysis: C 33 h 29 N 4 o 2 Na·H 2 O calculated value: C71.48 H5.10 N10.11
[0029] Experimental value: C71.42 H5.08 N10.22
Embodiment 2
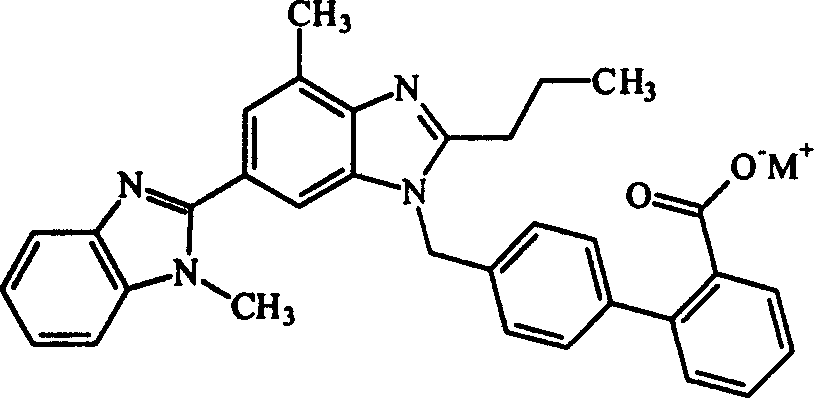
[0031] 4'-[(1,4'-dimethyl-2'-n-propyl[2,6'-di-1hydro-benzimidazol]-1'-yl)methyl]-[1,1' -Biphenyl]-2-carboxylate potassium salt
[0032] In a 250ml reaction bottle, add 10g (0.0195mol) Telmisartan (0.0195mol), 1.06g (0.0188mol) KOH and 100ml water, stir for 1 hour (30°C), filter, remove insoluble matter, concentrate to a small volume, add ethanol 30ml, concentrated, added 30ml of n-hexane to wash, poured off, added 30ml of ethanol, concentrated, repeated again, concentrated to dryness to obtain 10.6g of telmisartan potassium salt with a yield of 95.6%. Melting point: 203-205°C.
[0033] Elemental Analysis: C 33 h 29 N 4 o 2 K·H 2 O Calculated value: C69.04 H5.40 N9.76 Experimental value: C69.01 H5.28 N9.88
Embodiment 3
[0035] The fine powder of raw materials and auxiliary materials is evenly mixed and sieved, and 5% polyvinylpyrrolidone solution is added to make granules and dried. After the dry granules are sized, magnesium stearate is added, mixed evenly, and then compressed into tablets.
[0036] mg / tablet
[0037] Telmisartan sodium salt 20
[0038] Lactose 170
[0039] Sodium carboxymethyl starch 10
[0040] Meglumine 8
[0042] 5% polyvinylpyrrolidone solution appropriate amount
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com