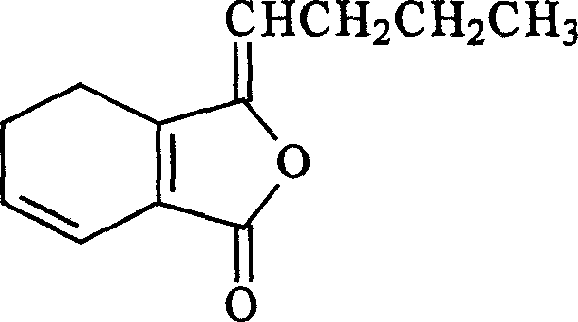
Medicinal use and extraction of cis-ligusticum lactone
A technology of ligustilide and an extraction method, applied in the field of medicinal use of cis-ligustilide, can solve problems such as inability to carry out industrialized production, and achieve the effects of improving blood flow state, high product yield, and resisting angina pectoris
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Crush 100Kg of Rhizoma Chuanxiong into fine powder with a particle size of 0.1-1mm. Add 1500kg of 95% ethanol to the extraction tank, start the high-shear instrument, and continuously add medicinal powder to the extraction tank for extraction. The speed of the high-shear instrument gradually increases from 2000 / min to 20000 / min, and the extraction temperature increases from room temperature to 20°C. Raise gradually, control it below 60 ℃, extract time is 3 hours. Collect the extract, recover the solvent under reduced pressure at 60°C, and apply the residue directly to a silica gel (200-400 mesh) column, elute with petroleum ether: ethyl acetate (98:2), collect the second color band, and store at 40 The solvent in the eluate was recovered under reduced pressure at ℃, and the residue was separated and purified repeatedly by the above-mentioned silica gel column chromatography method for 3 times. To remove the solvent, the temperature of the molecular distillation is 40°C...
Embodiment 2
[0039] Grind 115Kg of Rhizoma Chuanxiong into a fine powder of 0.1-1mm, put 800kg of ethyl acetate in the extraction tank, start the high-shear instrument, add medicinal powder continuously in the extraction tank for extraction, and gradually increase the speed of the high-shear instrument from 2000 / min. Rise to 20000 / min, extraction temperature is 20°C-60°C, extraction time is 1.5 hours, collect the extract, recover the solvent under reduced pressure at 50°C, and directly load the residue on a silica gel (200-400 mesh) column, use petroleum Ether: ethyl acetate (9:1) was eluted, the second color band was collected, and the solvent in the eluent was recovered under reduced pressure at 50°C, and the residue was separated and purified twice by the above silica gel column chromatography method, and the final After the eluent of the primary column chromatography recovers the solvent under reduced pressure, the residue is desolvated by molecular distillation. -6 Gradually rise to 1...
Embodiment 3
[0041] Crush 110Kg of Rhizoma Chuanxiong into a fine powder of 0.1-1mm, put 330kg of petroleum ether into the extraction tank, start the high-shear instrument, add medicinal powder continuously into the extraction tank for extraction, and gradually increase the speed of the high-shear instrument from 2000 / min To 20000 / min, the extraction temperature is controlled between 20-60°C, the extraction time is 0.5 hours, the extract is collected, the solvent is recovered under reduced pressure at 20°C, the residue is directly loaded on a silica gel (200-400 mesh) column, and the Petroleum ether: ethyl acetate (4:1) was eluted, the second color band was collected, and the solvent in the eluent was recovered under reduced pressure at 50°C. After removing the liquid and recovering the solvent under reduced pressure, the residue is removed from the solvent by molecular distillation. The temperature of the molecular distillation is 40°C, and the vacuum degree is increased from 10 -6 Gradua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com