Fire retardant in use for cellulose and fabricating method
A manufacturing method and technology for flame retardants, which are applied in the field of flame retardants and their manufacturing, can solve the problems of limiting the wide application of flame retardants, increasing product costs, and low flame retardant efficiency, and extending the flame retardant time and strengthening the flame retardant. Effect, strong flame retardant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
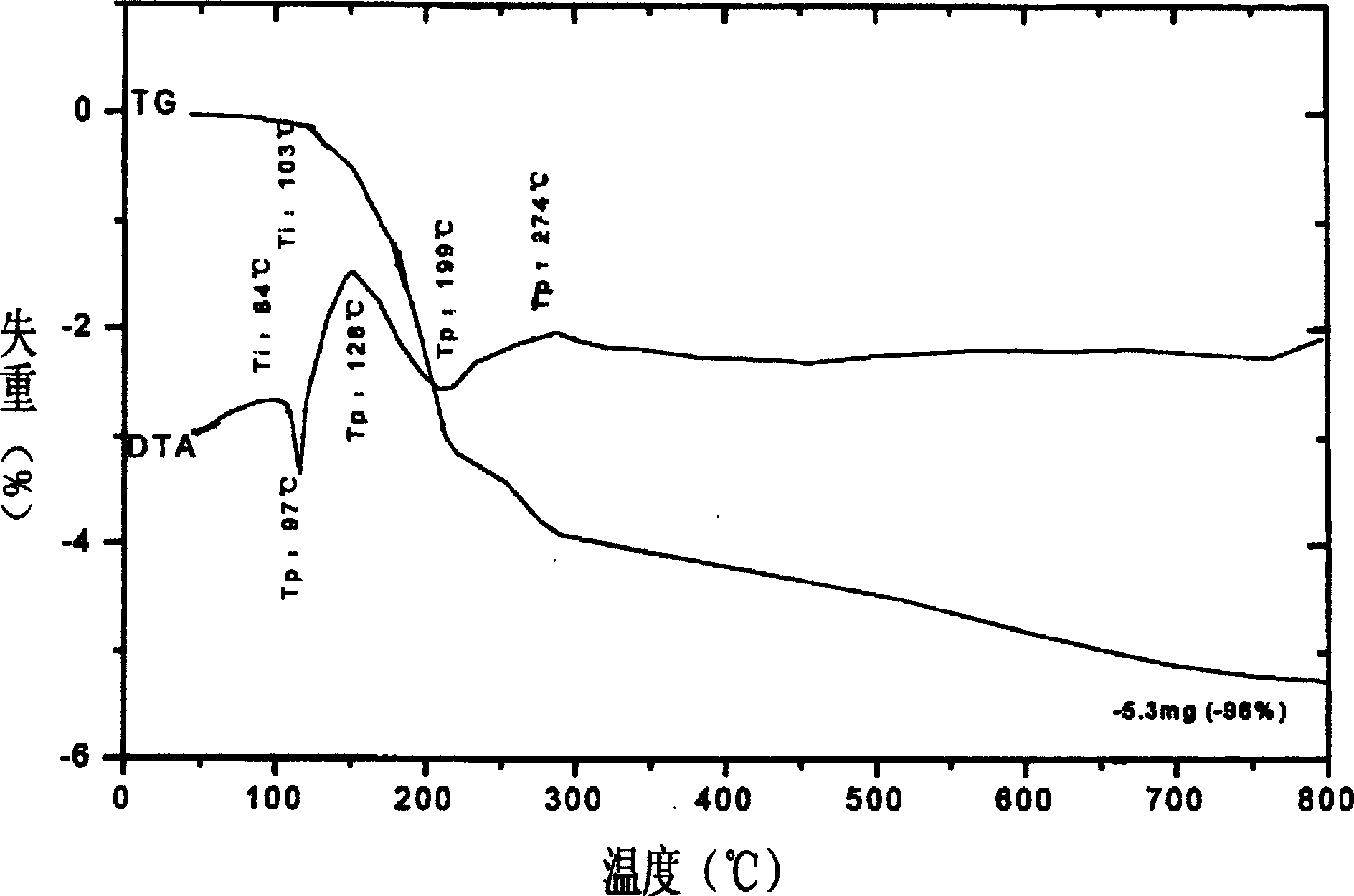
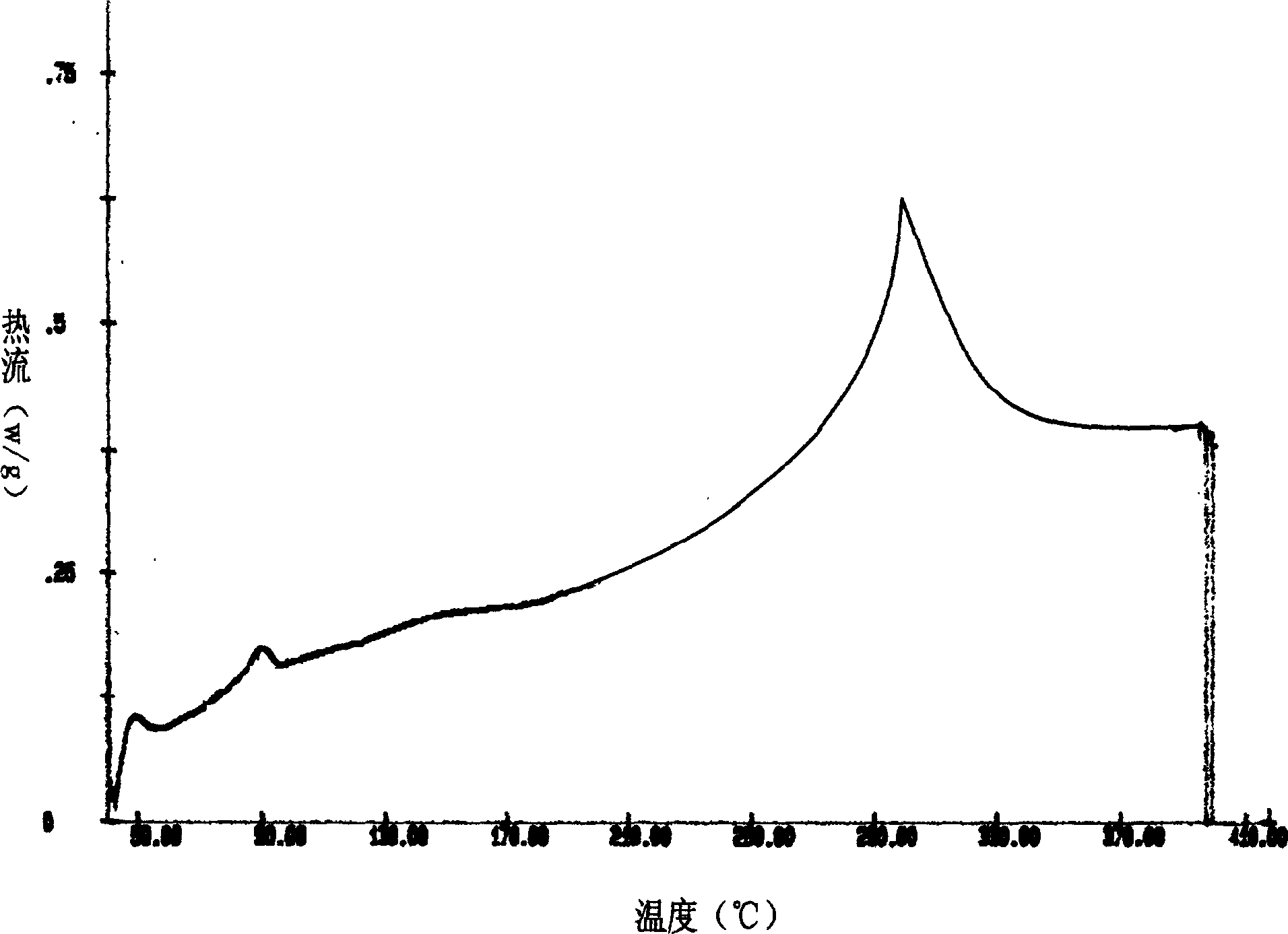
Image
Examples
Embodiment 1
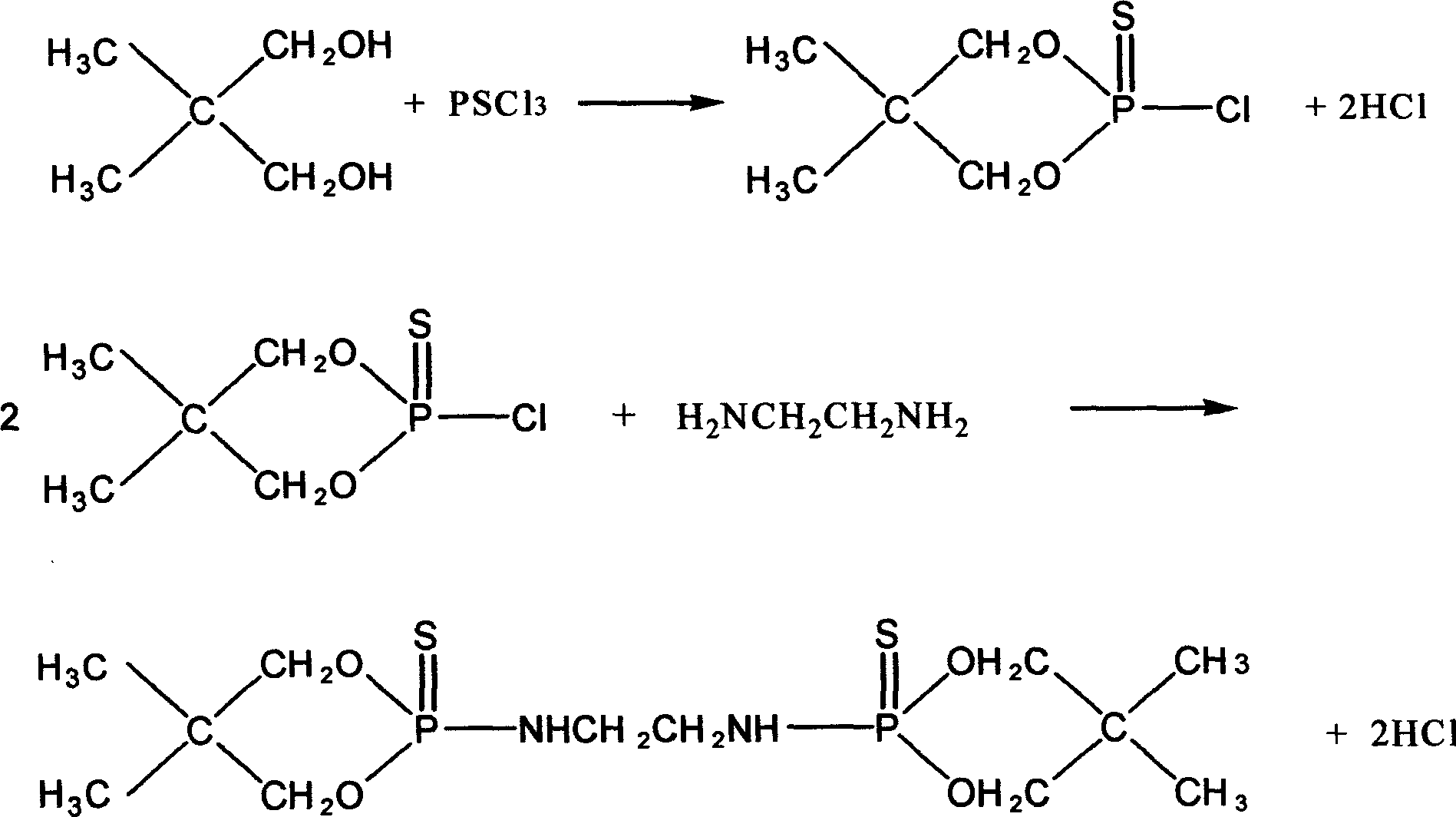
[0040] (1) PSCl 3 Synthesis
[0041] Add 1 mole of sulfur and 0.015 mole of aluminum trichloride to a four-necked flask equipped with a thermometer, agitator, and reflux condenser, and heat up to 115°C to melt the sulfur; Quickly add 1 mole of PCl to the four-neck flask 3 , the temperature was controlled at 145° C., and the reaction was carried out for 1 hour. Finally, the reaction solution is distilled, and the fraction between 120°C is taken to obtain a colorless and transparent liquid, which is PSCl 3 ;
[0042] (2) Synthesis of 2-thio-2-chloro-5,5-dimethyl-1,3,2-dioxaphosphorinane (DDSP)
[0043] Add 0.24 moles of PSCl to a four-necked flask equipped with a thermometer, a stirrer, and a reflux condenser 3and 0.24 moles of neopentyl glycol, and then added 0.05 moles of benzene, heated to 50°C to dissolve the neopentyl glycol, and then controlled the temperature at 45°C for constant temperature reaction for 1.5h. After the reaction, add 30ml of distilled water, shake w...
Embodiment 2
[0047] (1) PSCl 3 Synthesis
[0048] With embodiment 1;
[0049] (2) Synthesis of DDSP
[0050] Add 0.24 moles of PSCl to a four-necked flask equipped with a thermometer, a stirrer, and a reflux condenser 3 and 0.24 moles of neopentyl glycol, and then added 0.055 moles of benzene, heated to 50°C to dissolve the neopentyl glycol, and then controlled the temperature at 75°C for constant temperature reaction for 3 hours. After the reaction, add 30ml of distilled water, shake well and filter to separate the oil layer, and distill off benzene, and finally recrystallize the product with 0.15 mole of petroleum ether to obtain a white semi-crystalline solid, which is DDSP;
[0051] (3) Synthesis of DDPSN
[0052] Put 1 mole of DDSP after drying into a four-necked flask equipped with a thermometer, a stirrer, and a reflux condenser, and add 0.1 mole of CCl 4 , control the reaction temperature at 30°C, slowly add 0.5 mole of ethylenediamine, the reaction time is 3h, wash the reacti...
Embodiment 3
[0054] (1) PSCl 3 Synthesis
[0055] With embodiment 1;
[0056] (2) Synthesis of DDSP
[0057] Add 0.24 moles of PSCl to a four-necked flask equipped with a thermometer, a stirrer, and a reflux condenser 3 and 0.24 moles of neopentyl glycol, and then added 0.05 moles of benzene, heated to 50°C to dissolve the neopentyl glycol, and then controlled the temperature at 60°C for constant temperature reaction for 2.5 hours. After the reaction, add 30ml of distilled water, shake and filter to separate the oil layer, and distill to remove benzene, and finally recrystallize the product with 0.12 moles of petroleum ether to obtain a white semi-crystalline solid, which is DDSP;
[0058] (3) Synthesis of DDPSN
[0059] Put 1 mole of DDSP after drying into a four-necked flask equipped with a thermometer, a stirrer, and a reflux condenser, and add 0.1 mole of CCl 4 , control the reaction temperature at 25°C, slowly add 0.5 mole of ethylenediamine, the reaction time is 3h, wash the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com