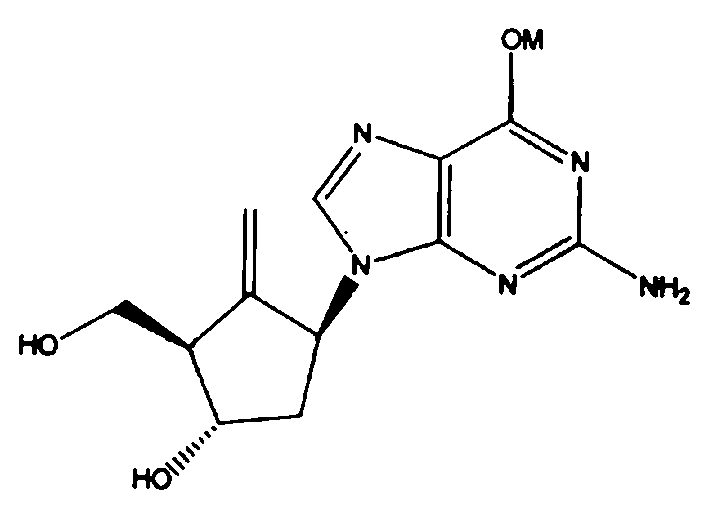
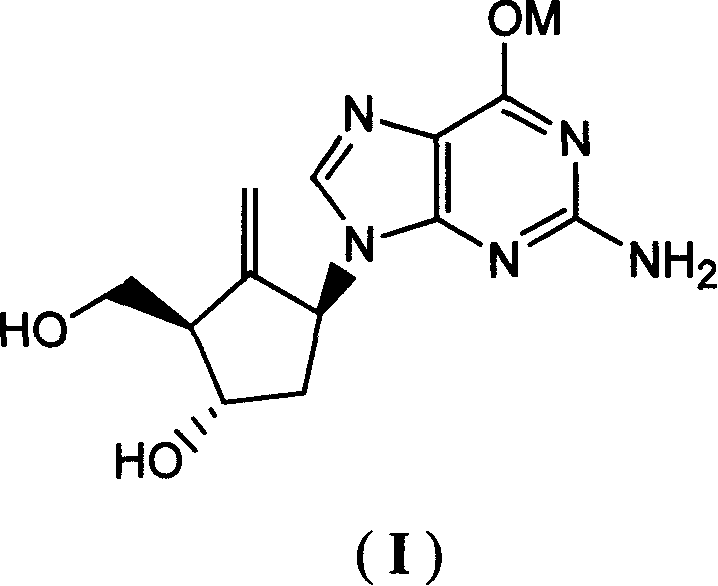
Antiviral activity possessed compound and preparation method thereof
A compound and composition technology, applied in the field of new antiviral compounds and their preparation, can solve the problems of cumbersome, long synthesis steps, harsh synthesis conditions, etc. The effect of viral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
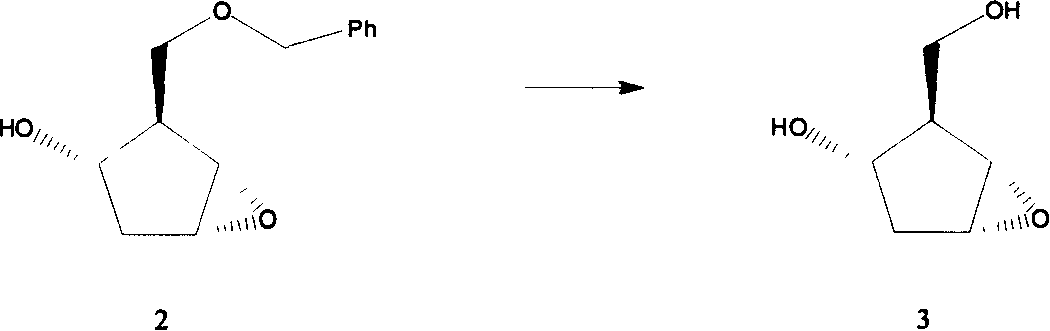
[0047] Example 1 Preparation of [1S-(1α, 2α, 3β, 5α)-2-hydroxymethyl]-6-oxabicyclo[3.1.0]hexan-3-ol (compound 3):
[0048] [1S-(1α, 2α, 3β, 5α)-2-[(benzyloxy)methyl]-6-oxabicyclo[3.1.0]hexan-3-ol 16g (67.8mmol) was dissolved in 0.5 g of 10% palladium carbon was added to 100 ml of tetrahydrofuran, and catalytic hydrogenation was carried out at room temperature for 8 hours. The catalyst was filtered off, and the solvent was evaporated to dryness under reduced pressure to obtain 9.8 g of pure compound 3.
Embodiment 2
[0049] Example 2 Preparation of compound 4:
[0050] Dissolve 9.5 g of [1S-(1α, 2α, 3β, 5α)-2-hydroxymethyl]-6-oxabicyclo[3.1.0]hexan-3-ol in 100ml of anhydrous acetone, then add 2 , 10 ml of 2-dimethoxypropane and a catalytic amount of p-toluenesulfonic acid. Stir at room temperature for 24 hours. The solvent was evaporated under reduced pressure, then dissolved in 100ml of dichloromethane, washed with saturated sodium bicarbonate (20ml×2), saturated brine (20ml×2), and dried over anhydrous sodium sulfate to obtain 11g of pure compound 4.
Embodiment 3
[0051] Example 3 6-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-benzyloxy--9H-purin-9-yl]-2,2-di Preparation of methylhexahydro-cyclopentyl[1,3]dioxan-5-ol (compound 5):
[0052] Under nitrogen protection, [2-[[(4-methoxyphenyl) diphenylmethyl]amino]-6-benzyloxy--9H-purine 36.5g and sodium hydride (60%) 2.85g Dissolve in DMF200ml. Stir at room temperature for 2 hours. Then, a solution of 10 g of compound 4 in 20 ml of DMF was added, the temperature was raised to 120° C., and the mixture was stirred for 10 hours. After concentration under reduced pressure, the residue was chromatographed on a silica gel column and eluted with petroleum ether-ethyl acetate (2:1) to obtain 29.3 g of pure compound 5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com