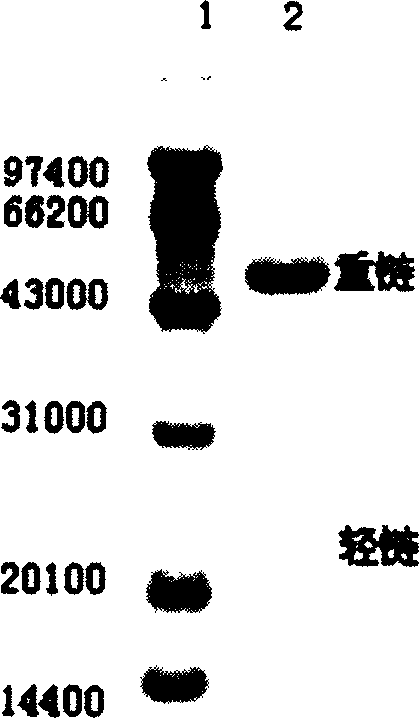Antigen epitope of gold staphylococcus virulence factor regulatory protein and its mimic epitope and use thereof
A technology of Staphylococcus aureus virulence factor and regulatory protein, applied in the field of two antigenic epitopes and their mimetic epitopes, can solve the problems of inability to determine antigenic epitopes, difficult to carry out research, difficult and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Preparation and purification of TRAP protein polyclonal antibody
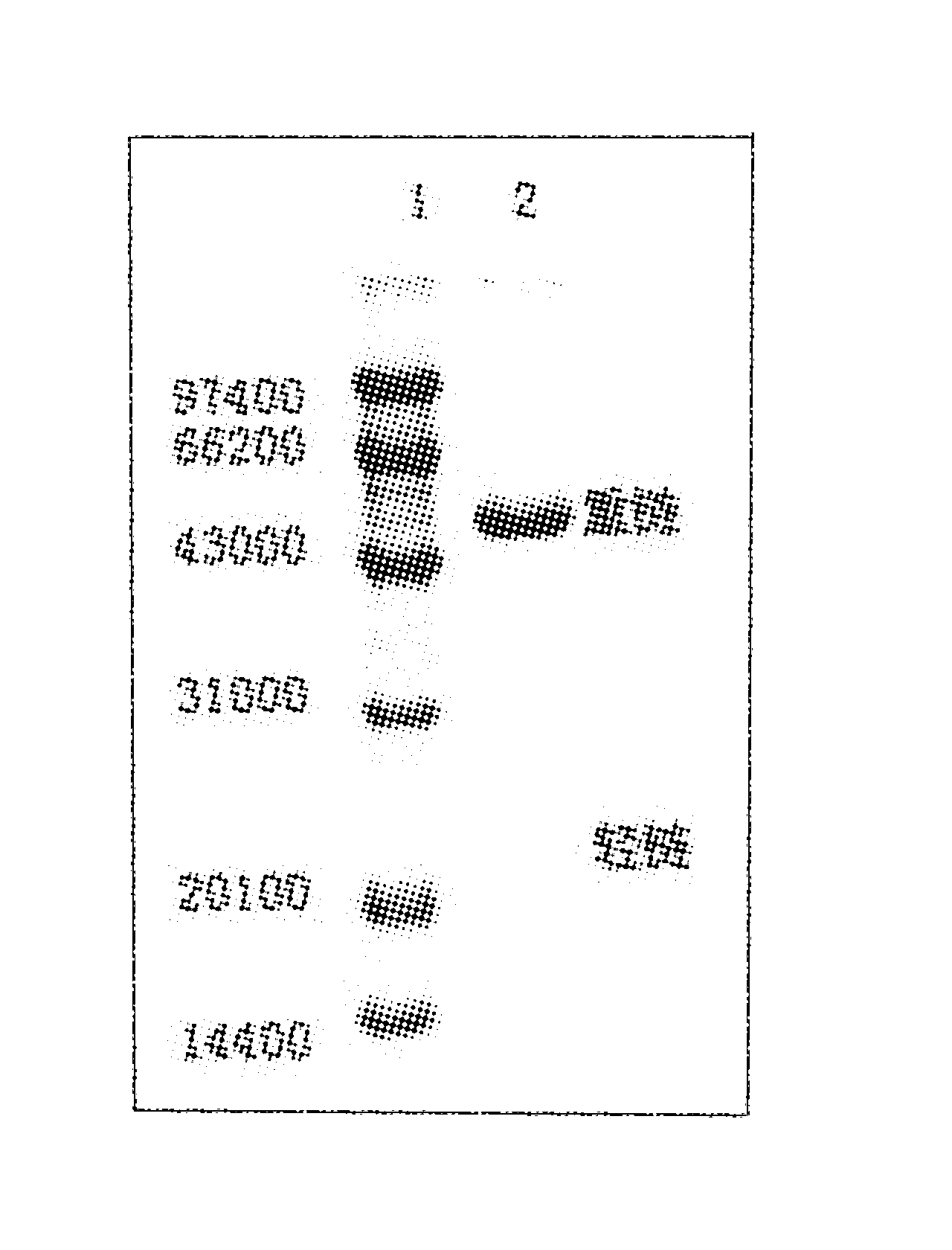
[0027] The purified TRAP protein was immunized to rabbits to prepare polyclonal antibodies. An affinity column coupled with TRAP protein was used to separate anti-TRAP IgG from polyantibody serum. SDS-PAGE electrophoresis results showed that the purity of the purified IgG was >90%, and the ELISA results showed that the titer was 1×10 5 Above (see attached figure 1 In the figure, 1 represents the low molecular weight protein standard, and 2 represents the purified TRAP polyclonal antibody).
Embodiment 2
[0028]Example 2. Screening of mimotopes of TRAP polyclonal antibody
[0029] First, 100μl of purified TRAP polyclonal IgG was coated on the enzyme-linked plate and placed at 4°C overnight. After blocking with 2% gelatin for 1 hour, add the phage dodecapeptide library, incubate at room temperature for 1 hour, wash the non-specifically bound phage with TBST (50mmol / LTris-HCl, 0.1% TWEEN20, pH7.5), and then use 0.2mmol / L Glycine-HCl pH2.2 eluted the specifically bound phage, and the eluate was neutralized with 1mmol / L Tris-HCl pH9.0. According to the method provided by the kit, determine the titer of the phage in the eluate, and use the titer of the eluted phage of the uncoated target protein as a control to determine the input-output ratio. At the same time, the eluted phage bound to TRAP antibody IgG was amplified, and its titer was measured for the next round of screening. After 3 rounds of screening, the measured input and output have been significantly improved. The enriched ph...
Embodiment 3
[0030] Example 3. Comparison of the screened antigen mimic epitope sequence 1 and the expressed TRAP sequence
[0031] Analyzing the screened sequence and the original sequence of the expressed TRAP protein, it is found that the sequence of the antigen mimic epitope sequence 1 is similar to the amino acid sequence 21-34 of the TRAP protein:
[0032] Antigen mimotope sequence 1 NP LH HE HA TG W T
[0033] TRAP protein primary sequence 21 NP T HO LFQF SA SD T 34
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com