Light cut-out magnetic particle molecale label, its preparation method and aplication in multipeptide synthesis preparation
A technology of molecular labeling and magnetic particles, which is applied in the application fields of large-scale synthesis and purification of polypeptides, can solve the problems of no discovery, achieve low cost, make up for the defects and deficiencies of process methods, and save the investment in instruments and reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of Magnetic Particle Molecular Tags
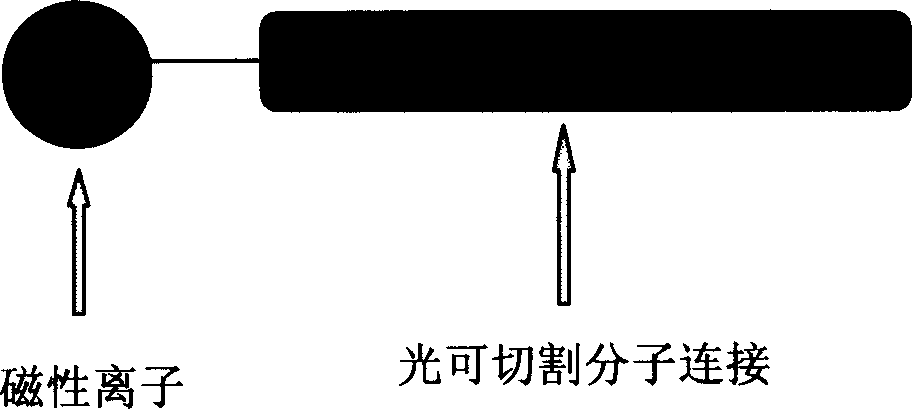
[0042] Weigh 1 gram of Fe 3 o 4 Magnetic particles, the outer periphery of the magnetic particles has been coated with NH 2 terminal, terminal NH 2 The molar number is 1mmol / g, weigh 2mmol of photocleavable linker molecule 4-Hydroxymethyl-2-methoxy-5-nitrophenoxybutyri Acid (4-hydroxymethyl-2-methoxy-5-nitrophenoxybutyric acid) , using anhydrous HOBT, DIEA, 4-fold molar excess, in 5ml DMF solution system, activate photoremovable molecules, add the liquid activated for 5-8 minutes to the magnetic particles, and react in the dark for 2.5 hours, the reaction is complete , using magnet adsorption and precipitation to precipitate the molecular tags linked by reaction, washing with DMF, DCM, and methanol respectively and precipitating three times, drying under reduced pressure for later use, and obtaining 1.32 g of linked magnetic particle molecular tags.
Embodiment 2
[0043] Embodiment 2: synthetic preparation 0.5mmol P23 peptide
[0044] The synthetic preparation sequence is SKYTESFVAAFKRAGAGVEKAEA-NH 2 , a polypeptide with a sequence length of 23 amino acids.
[0045] Weigh 0.5 grams of Wang resin with a degree of substitution of d=1mmol / g, add it to the polypeptide reactor, and assemble and synthesize the polypeptide using the standard Fmoc coupling method. After each step of amino acid connection, use excess acetic anhydride according to the ratio of acetic anhydride:DMF=1:1 (V / V), 4-6mL blocked unreacted NH 2 end, to prevent the continued assembly and synthesis of the truncated sequence, and cut off the terminal NH after the last amino acid is synthesized 2 protection of.
[0046] Wash well to keep the peptide-resin moist, weigh 2.5 grams of the magnetic particle molecular label prepared in Example 1, and use HOBT, PyBOP (benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate, hexafluorophosphate-1-oxyl Tripyrrolidinyl ...
Embodiment 3
[0047] Example 3 Synthetic preparation of 1 mmol P38 peptide
[0048] The synthetic preparation sequence is HSDGIFTDSYSRYRKQMAVKKYLAAVLGKRYKQRIKNK-NH 2 A polypeptide with a sequence length of 38 amino acids
[0049] Weigh 0.5 grams of Rink Amide-AM resin with a degree of substitution of d=0.3-0.8mmol / g and add it to the polypeptide reactor, assemble and synthesize the polypeptide using the standard Fmoc coupling method, and use excess acetic anhydride to press acetic anhydride after each step of amino acid connection: DMF=1:1 (V / V), 4-6mL blocked unreacted NH 2 end, to prevent the continued assembly and synthesis of the truncated sequence, and cut off the terminal NH after the last amino acid is synthesized 2 2.5 g of the magnetic particle molecular tag prepared in Example 1 was weighed, and 4 times the molar amount of HOBT, PyBOP, and DIEA was used as a catalyst to activate the molecular tag in 5 ml of DMF to activate the 5- After 8 minutes, add the peptide-resin to the re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com