Loratadine paster of penetrating skin
A technology of transdermal patch and loratadine, which is applied in the field of penetration-enhancing technology and medicine, can solve the problem that the penetration performance of loratadine cannot fully meet the treatment requirements, and achieve flexible and individualized drug administration , good in vivo transdermal rate and stable blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Selection of basic prescription
[0044]According to the use of polyacrylate polymer material Eudragit E100 as the skeleton and adhesive, succinic acid as the crosslinking agent, dibutyl sebacate as the plasticizer, and ethanol as the solvent.
[0045] The fixed dosage of EUDRAGIT E 100 in the prescription of the present invention is 1.8 g, and the calculation of the dosage of dibutyl sebacate and succinic acid is based on EUDRAGIT E 100, and the percentages of the two in EUDRAGIT E 100 are calculated. The amount of cross-linking agent and plasticizer is selected according to the product description of succinic acid, dibutyl sebacate, polymer material Eudragit E100, the effective range of succinic acid is 2~18%, and the effective range of dibutyl sebacate is effective The range is 20~70%.
[0046] The prescription was optimized according to the orthogonal experiment design. From this, determine the level of each factor, see Table 1.
[0047] Table 1 Orthogonal tes...
Embodiment 2
[0076] Example 2: Basic prescription study of loratadine transdermal patch
[0077] Composition of prescription: prescription 1
[0078] Loratadine 36mg
[0079] Eudragit E 100: 1800mg
[0080] Succinic acid: 120mg
[0081] Dibutyl sebacate: 0.85ml
[0082] Ethanol: 3ml
[0083] Weigh Eudragit E 100, succinic acid, and dibutyl sebacate according to the prescription ratio, add ethanol, and let it stand for 24 hours to fully swell Eudragit E 100. Add loratadine according to the prescription ratio and let it stand for several hours; It is made of medicine-containing glue, ultrasonically degassed, and coated on anti-sticking paper with a thickness of 0.1mm, plus a backing layer, and dried.
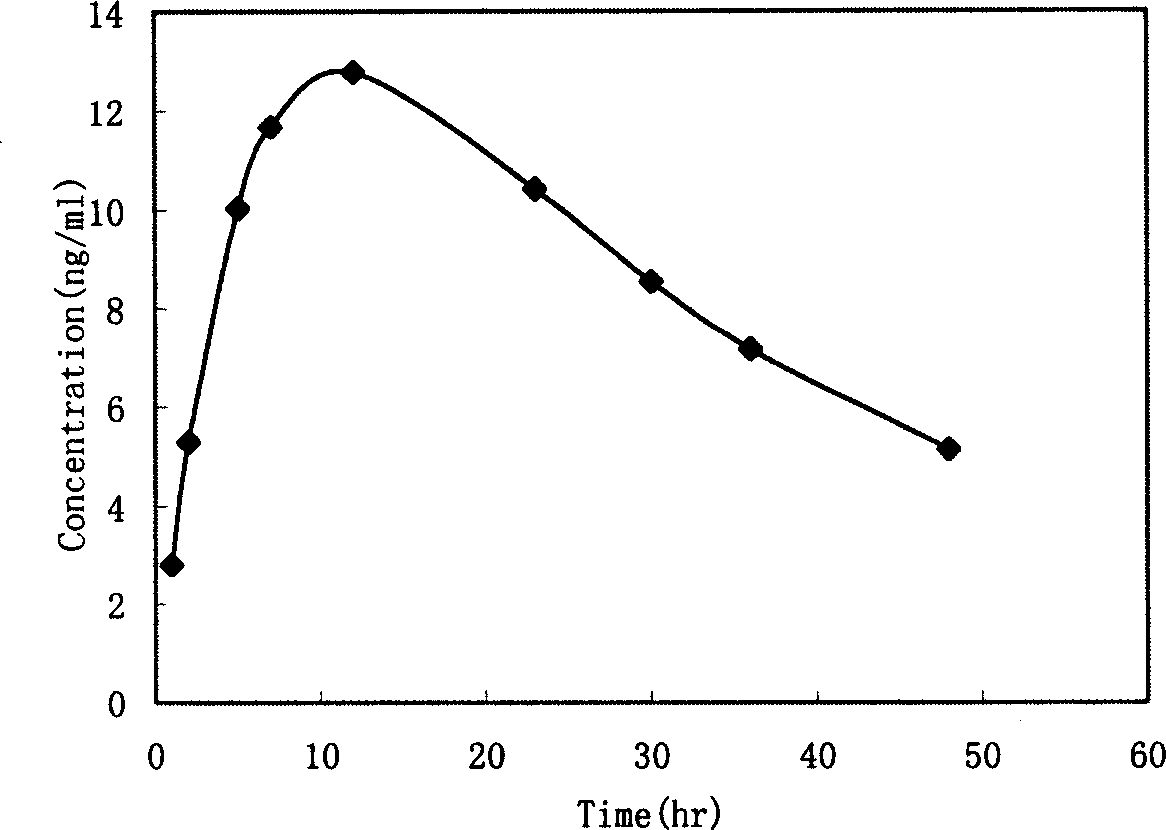
[0084] Cut the patch into a small round piece with a diameter of 1.9cm, place it on a transdermal diffusion tester, perform a transdermal absorption test, measure the cumulative transdermal amount Q (μg) at different times, and measure the penetration a...
Embodiment 3
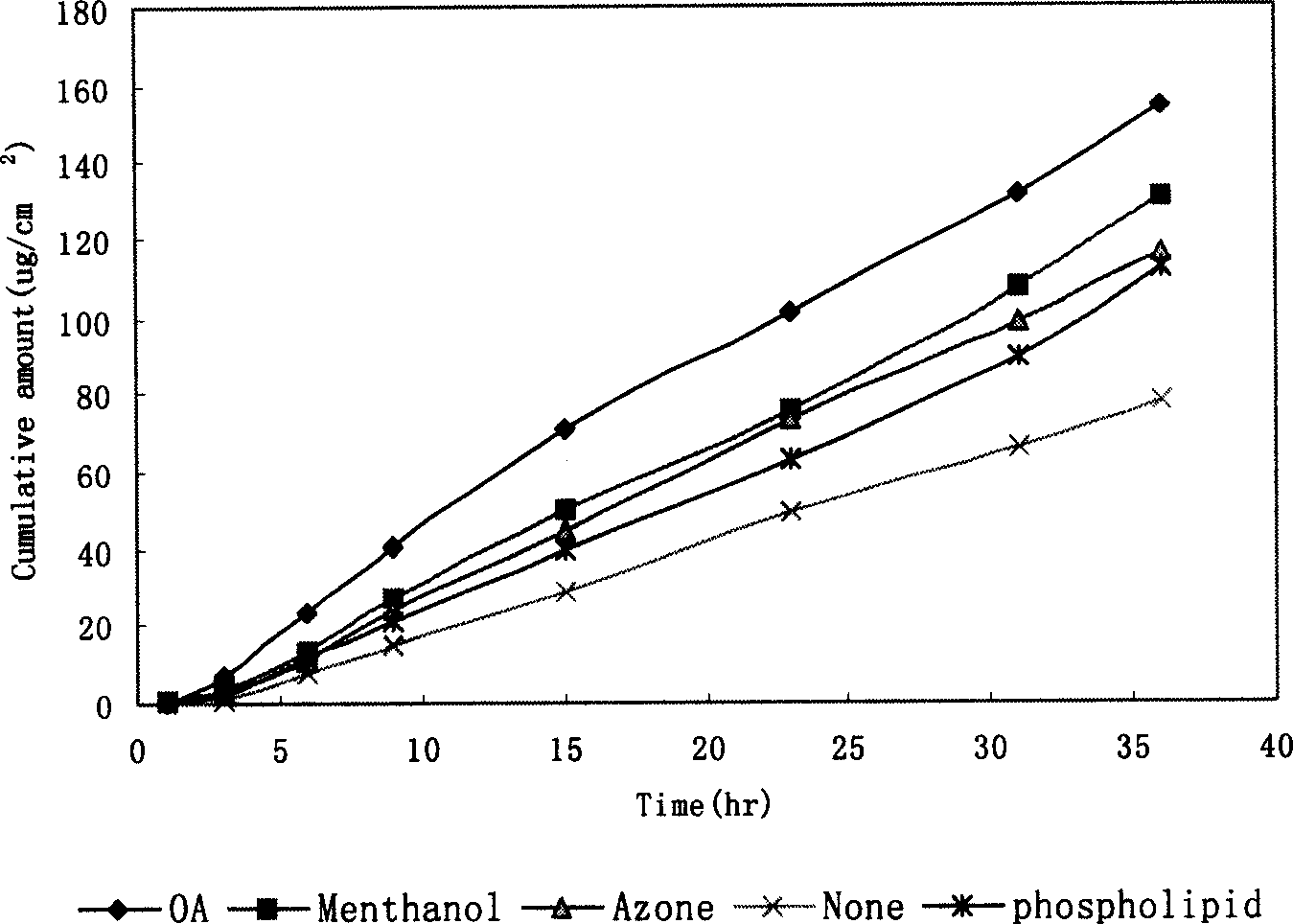
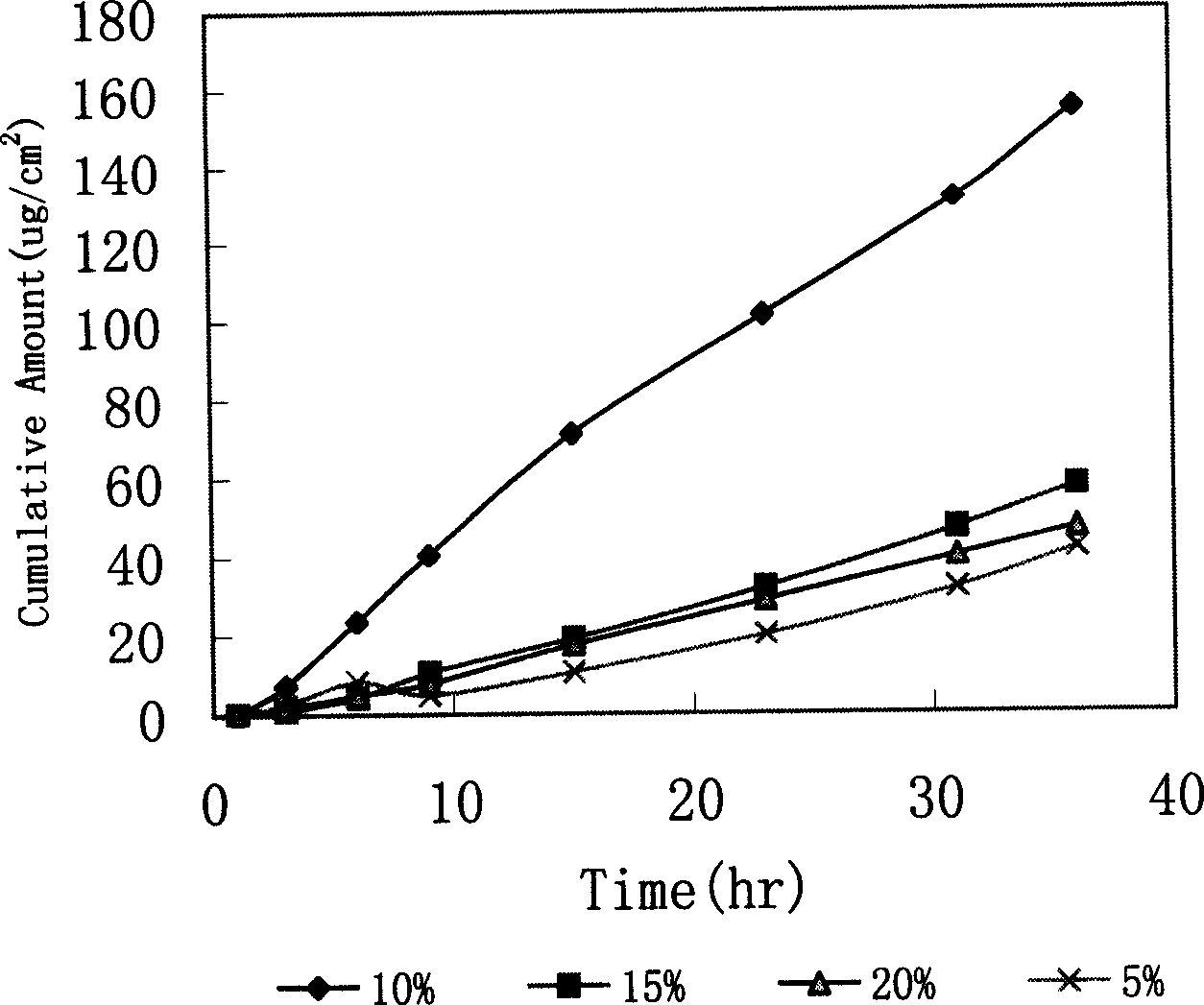
[0087] Example 3: Study on the penetration promoting effect of oleic acid on loratadine
[0088] Composition of prescription: prescription 2 prescription 3 prescription 4 prescription 5
[0089] Loratadine 36mg 36mg 36mg 36mg
[0090] EudragitE100: 1.8g 1.8g 1.8g 1.8g
[0091] Succinic acid: 120mg 120mg 120mg 120mg
[0092] Dibutyl sebacate: 0.85ml 0.85ml 0.85ml 0.85ml
[0093] Oleic acid: 5%(0.15ml) 10%(0.3ml) 15%(0.6ml) 20%(0.9ml)
[0094] Ethanol: 3ml 3ml 3ml 3ml
[0095] Weigh Eudragit E 100, succinic acid, and dibutyl sebacate according to the prescription ratio, add ethanol, and let stand for 24 hours to make Eudragit E 100 fully swell, add loratadine according to the prescription ratio accurately, and add different proportions of oil. Acid, let it stand for several hours; fully stir and mix to form a drug-containing glue, ultrasonically degas, and coat it on a release paper with a thickness of 0.1mm, add a backing layer, and dry it.
[0096] Cut the patch into small round p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com