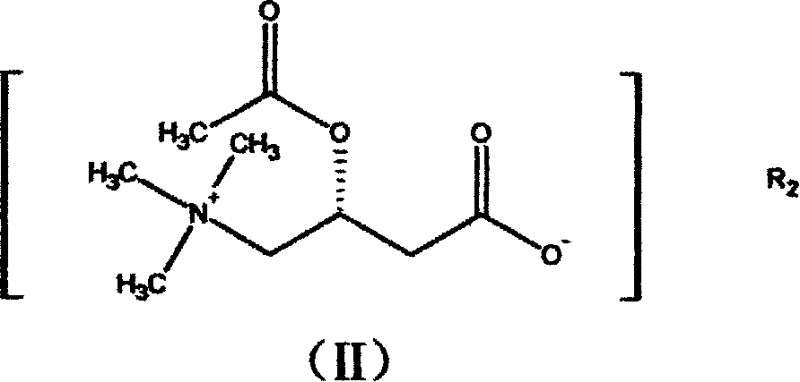
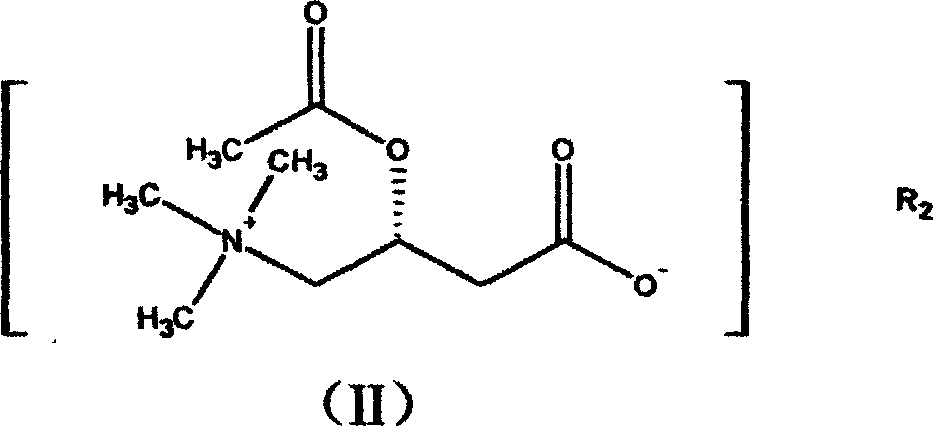
Application of levocarnitine and its derivative in preventing and treating high altitude diseases
A derivative, high-altitude sickness technology, applied in the field of prevention and treatment of high-altitude sickness, to achieve the effect of promoting economic development, broadening the scope of application, and expanding the living space of human beings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Prepared levocarnitine tablet according to the following formula (all contents used are weight percentages)
[0031] L-Carnitine Tartrate 50%
[0032] Lactose 25%
[0033] Starch 24%
[0034] Stearic acid 1%
[0035] (1) Pulverize levocarnitine tartrate and lactose respectively, pass through a 100-mesh sieve, and directly pass the starch through a 100-mesh sieve for subsequent use;
[0036] (2) Take an appropriate amount of starch to prepare 10% starch paste for use as a binder;
[0037] (3) Mix levocarnitine, lactose and the remaining amount of starch, add a binder to make soft powder, pass through a 16-mesh sieve to make granules;
[0038] (4) Place the wet granules in a drying oven at about 50°C, and dry them by air for about 3 hours;
[0039] (5) After the dry granules are sized through a 16-mesh sieve, magnesium stearate is added and mixed uniformly;
[0040] (6) Punch and press the tablet with a diameter of 90 mm.
Embodiment 2
[0042] L-carnitine tartrate capsules were made according to the following formula (all contents used are weight percentages)
[0043] L-Carnitine Tartrate 90%
[0044] Starch 10%
[0045] (1) L-carnitine tartrate and starch are respectively passed through a 100 mesh sieve for pretreatment;
[0046] (2) Fully mix the processed L-carnitine tartrate and starch;
[0047] (3) Fill the mixed medicinal powder into No. 2 empty capsules.
Embodiment 3
[0049] Levocarnitine oral liquid was obtained according to the following formula (all contents used are weight percentages)
[0050] L-Carnitine Inner Salt 5%
[0051] Stabilizer 94%
[0052] Citric acid 1%
[0053] Appropriate amount of preservatives
[0054] Add distilled water, aliquot into 5g / 100ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com