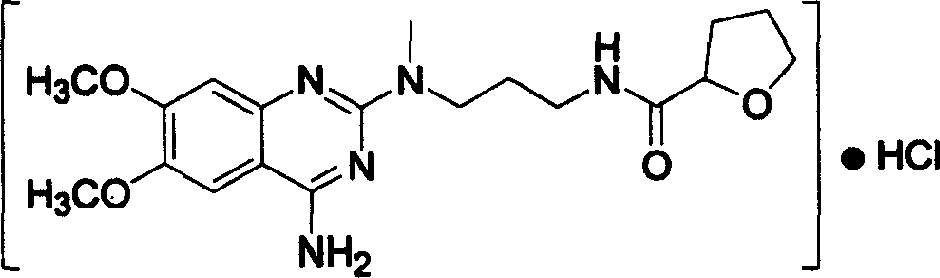
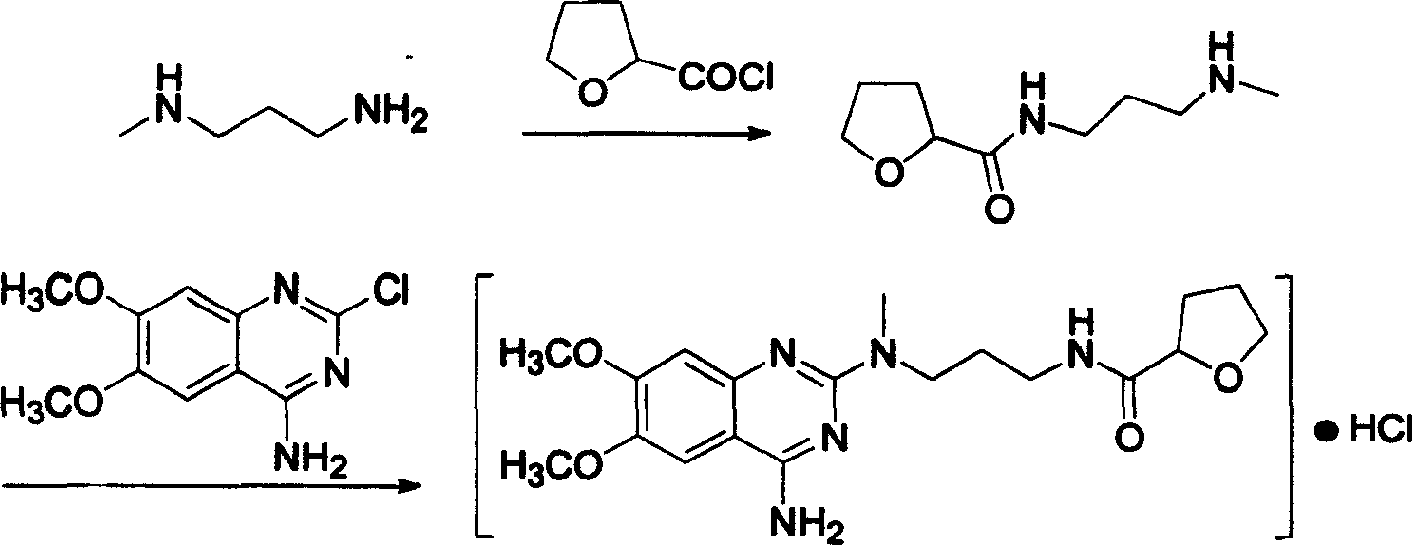
Process for preparing alfuzosin hydrochloride
A technology for alfuzosin hydrochloride and an organic base, which is applied in the field of preparation of alfuzosin hydrochloride, can solve problems such as difficulty in realizing large-scale industrial production of alfuzosin hydrochloride, and achieves easy realization of reaction conditions, simplified operation, and reduced The effect of three waste pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the preparation of alfuzosin hydrochloride
[0017] In a 3000 ml three-neck flask with mechanical stirring, add 1800 ml of chloroform and 82 ml of triethylamine, stir, cool down to 0°C in an ice-water bath, add 88 g of N-methylpropylenediamine and 134.5 g of 2-tetrahydrofuran Formyl chloride was reacted at room temperature for 18 hours, then 245.5 g of 2-chloro-4-amino-6,7-dimethoxyquinazoline was added, the temperature was controlled at 35-40°C, and the reaction was continued for 12 hours. After the reaction, wash with 1000 ml × 2 water, 800 ml 5% NaHCO 3 Washing with aqueous solution, washing with 800 ml saturated brine, anhydrous Na 2 SO 4 dry. After the solvent was evaporated under reduced pressure, it was dissolved in 1500 ml of absolute ethanol, and 380 ml of absolute ethanol-hydrogen chloride solution was added, stirred evenly, and placed for crystallization. Suction filtration and drying to obtain a white crystalline solid, which was recrystall...
Embodiment 2
[0018] Embodiment 2: the preparation of alfuzosin hydrochloride
[0019] In a 3000 ml three-necked flask with mechanical stirring, add 1600 ml of DMF (N, N-dimethylformamide) and 98 ml of pyridine, stir with 5 g of DMAP, cool to 0 ° C, add 88 g of N-methyl Propylenediamine and 104.5 grams of 2-tetrahydrofurancarboxylic acid were reacted at room temperature for 20 hours, and 245.5 grams of 2-chloro-4-amino-6,7-dimethoxyquinazoline were added. Control the temperature at 35-40°C and react for 14 hours. After completion of the reaction, wash with water (1000 ml × 2), 5% NaHCO 3 Wash with 800 ml of aqueous solution, wash with 800 ml of saturated saline, and wash with anhydrous Na 2 SO 4 dry. After evaporating the solvent under reduced pressure, dissolve it with 1500 ml of absolute ethanol, add 180 ml of absolute ethanol-hydrogen chloride, stir evenly, place to crystallize, filter with suction, and dry to obtain 226 grams of white crystalline solid, which is the fine product of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com