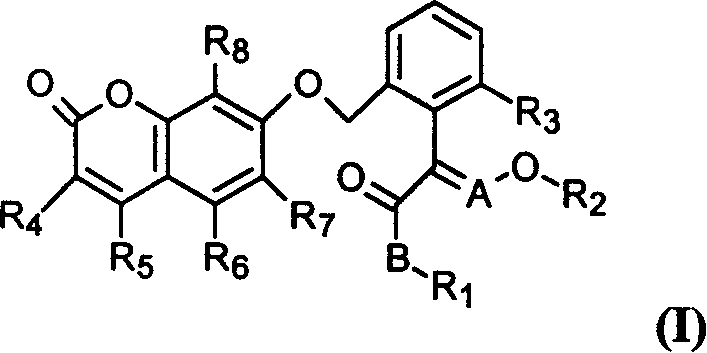
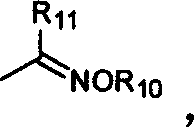
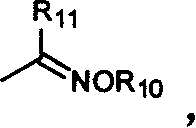
Benzopyrone compounds with pest killing and sterilizing activity and preparation and use
A technology of benzopyrone and compounds, applied in the fields of application, organic chemistry, botany equipment and methods, etc., can solve the problem of low activity of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0094] Example 1: The preparation method of compound 1
[0095] At room temperature, 0.84 g of 60% sodium hydride was added to the reaction flask, washed with petroleum ether, 30 ml of dry N, N dimethylformamide was added thereto, the reaction was stirred for half an hour, and 1.7 g of 7 -Hydroxycoumarin, continue to stir until no gas is released, add 3 grams of (E)-2-[2-(bromomethyl)phenyl]-3-methoxymethyl acrylate, and continue to stir for 3 hours. The reaction mixture was poured into ice water, extracted three times with ethyl acetate, the combined extracts were washed three times with saturated brine, dried, filtered, and concentrated under reduced pressure to obtain 5 g of oily liquid. Column chromatography gave 2.8 g of the title compound as a light reddish-yellow oil with a yield of 76.5%.
example 2
[0096] Example 2: Preparation of compound 2
[0097] At room temperature, 0.45 g of 60% sodium hydride was added to the reaction flask, washed with petroleum ether, 20 ml of dry N,N dimethylformamide was added thereto, the reaction was stirred for half an hour, and 1.0 g of 7 -Hydroxy-4-methylcoumarin, continue to stir until no gas is released, add 1.66 grams of (E)-2-[2-(bromomethyl)phenyl]-3-methoxymethyl acrylate, continue to stir 3 hours. The reaction mixture was poured into ice water, extracted three times with ethyl acetate, the combined extracts were washed three times with saturated brine, dried, filtered, and concentrated under reduced pressure to obtain a yellow solid as a crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:2) gave 1.73 g of the title compound with a melting point of 140-143°C. Yield 80%.
example 3
[0098] Example 3: Preparation of compound 101
[0099] At room temperature, containing 1.2 grams of anhydrous potassium carbonate, 1.0 grams of 7-hydroxy-4-methylcoumarin, 1.70 grams of 2-bromomethyl-α-(methoxyimino) methyl phenylacetate in 20 The mixed solution of butanone in milliliters was heated under reflux and stirred for 5 hours, the reaction mixture was poured into ice water, extracted 3 times with ethyl acetate, the combined extracts were washed 3 times with saturated brine, dried, filtered, and concentrated under reduced pressure to obtain a yellow solid as crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:2) gave 1.77 g of the title compound, melting at 150-152°C. Yield 83%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com