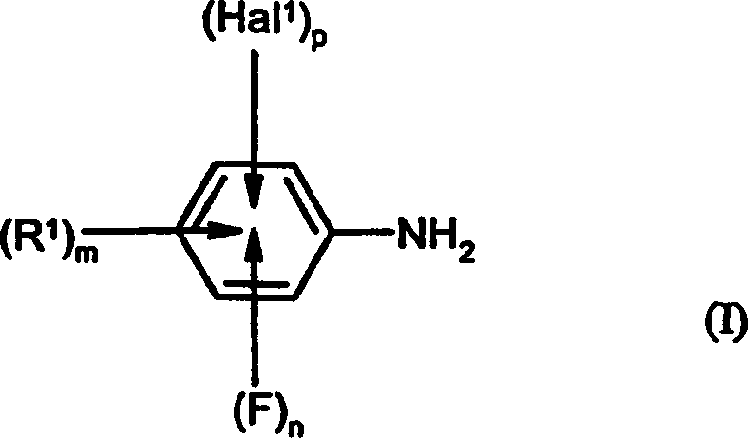
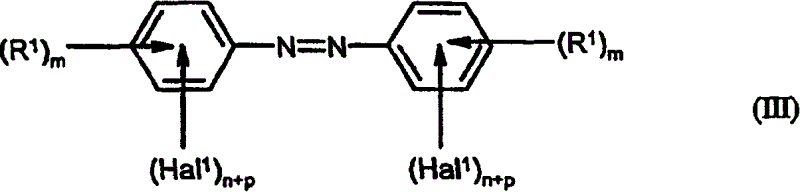
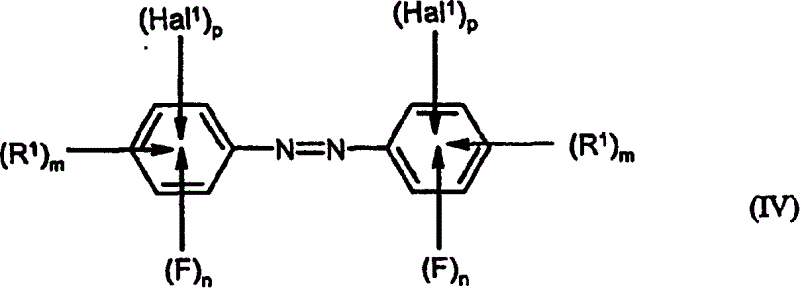
Preparation of fluorinated anilines
A compound, azobenzene technology, applied in the field of preparing fluorinated aniline, can solve problems such as unusable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Preparation of 2,2',4,4',6,6'-hexafluoroazobenzene
[0077] In the absence of moisture, initially 4.3 g of dry potassium fluoride and 3.0 g of 2,2',4,4',6,6'-hexachloroazobenzene were added to 51 g of sulfolane, and 0.2 g of bromide Tetraphenylphosphonium. Subsequently, the mixture was heated to 180° C. and stirred for 12 hours. After cooling, the mixture was drained into 150 ml of water and extracted twice with 50 ml of cyclohexane (each). After crystallization, 1.7 g (76% of theory) of the desired product were obtained.
Embodiment 2
[0079] Preparation of 2,4,6-trifluoroaniline
[0080] 2,2',4,4',6,6'-hexafluoroazobenzene of 1.0g Example 1 was dissolved in 20ml methanol at room temperature, and 0.6g ammonium formate and 0.5g zinc powder were added continuously. The mixture was stirred at room temperature for 1 hour. The starting material was completely converted by GC analysis. After methanol had been distilled off, 2,4,6-trifluoroaniline was extracted in cyclohexane and dried, and the solvent was distilled off again. This leaves 0.9 g of the desired product (89% of theory).
Embodiment 3
[0082] Preparation of 2,2'-bis(trifluoromethyl)-4,4'-difluoroazobenzene
[0083] In the same manner as in Example 1, 9.8 g of 2,2'-bis(trifluoromethyl)-4,4'-dichloroazobenzene, 4 g of potassium fluoride and 0.5 g tetraphenylphosphonium bromide was reacted for 15 hours. After crystallization, 5.8 g of 2,2'-bis(trifluoromethyl)-4,4'-difluoroazobenzene (65% of theory) were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com