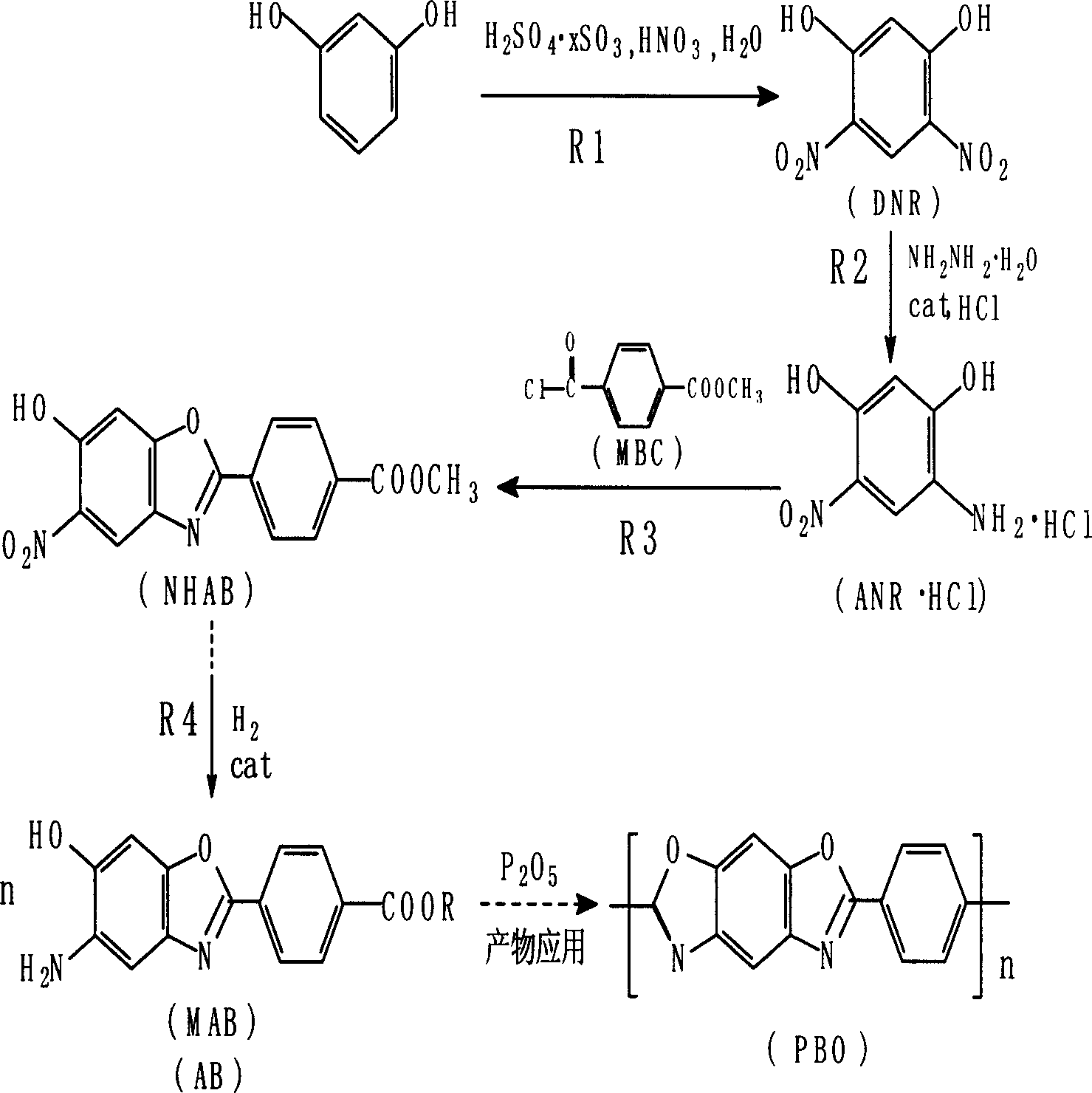
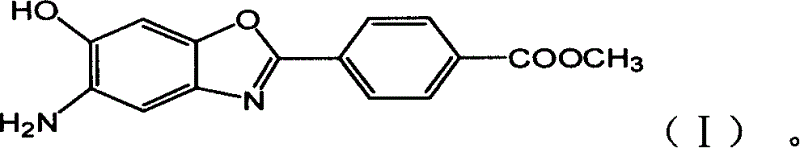
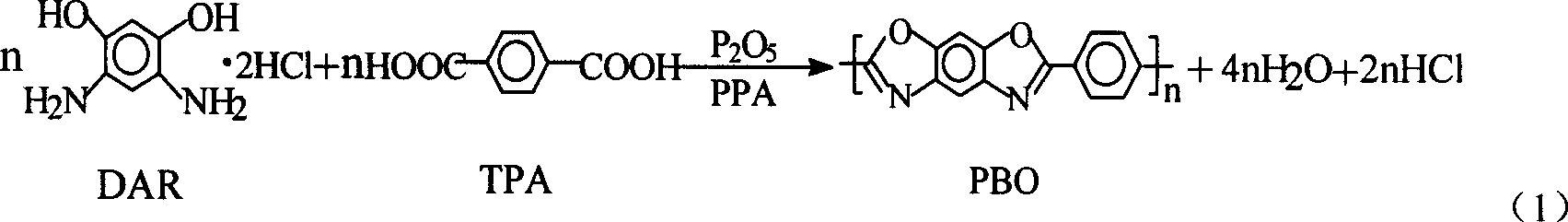
AB type poly(p-phenyl) benzdioxan monomer and its preparation and use
A technology of benzobisoxazole and benzoxazole, which is applied to the application field in the preparation of PBO resin, can solve the problems of high price, difficulty in solving DAR, and high operation cost of PBO preparation, and achieves easy operation, cost reduction and operation. handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of 5-nitro-6-hydroxyl-2-(p-methoxycarbonylphenyl)benzoxazole (NHAB)
[0037] Take 15.18g 4-amino-6 nitroresorcinol hydrochloride (ANR HCl, 0.073mol) plus 13.57g p-methoxycarbonylbenzoyl chloride (MBC, 0.068mol) and add 120ml of solvent diethylene glycol dimethyl In ether, heat up to dissolve, react at 122-128°C for 2 hours, add 47.5g of polyphosphoric acid and then heat up to 156-160°C for about 3 hours, then add water for water analysis, filter, wash the filter cake with water, and dry to obtain the crude product of NHAB 20.85 g, HPLC purity 89.61%, crude product yield 90.33%; 14-dioxane with 10 times the crude product quality was selected for reflux decolorization and recrystallization to obtain 14.05g of NHAB fine product with a purity of 98.62%, a refined yield of 54.96%, and a melting point of : 234.8-235.5°C. IR (KBr, cm -1 ): 3320~3110(b, m), 3106.8(m), 1724.0(s), 1545.7(s), 1440.6(s), 1332.6(s), 1283.4(s), 708.7(s). 1 H NMR (500MHz CDCl...
Embodiment 2~8
[0042] Embodiment 2~8: the preparation of NHAB
[0043] For the synthesis of NHAB, according to the difference between the prepared ANR and ANR·HCl, the two monomers were used to test respectively. Adopt the same operation of embodiment 1, get different parameters to test by the parameter scope of the present invention, the results are shown in Table 1:
[0044] implement
[0045] The results show that: the NHAB synthesized by ANR as the raw material has a refined product yield of 47.10% and a refined purity of 95.22%; while the NHAB synthesized by ANR·HCl as a raw material has a purity of 85.44%, a yield of 87.32%, and a refined purity of 96.98%. Yield 52.71%; Visible synthetic NHAB is still better to adopt ANR HCl as raw material, it has higher yield than ANR raw material likewise, but the yield of NHAB especially when excessive MBC proportioning and lower PPA consumption Decrease faster; and the purity of NHAB obtained is not high, generally all about 90%, so the...
Embodiment 9~13
[0046] Embodiment 9~13: the refinement of NHAB
[0047] Adopt the same operation of embodiment 1 to refine and purify the NHAB crude product, get the following two methods of different parameters (two kinds of solvents and proportioning) by the parameter scope of the present invention to carry out purification effect test, simultaneously to adopting the DMF outside the present invention The refining invalidity result of solvent is carried out comparative test, and the results are shown in Table 2:
[0048] implement
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com