Toad extract liposome preparation for injection and its preparing method
A technology of liposome preparation and extract, which is applied in the field of pharmaceutical preparations for injection, can solve the problems of low effective concentration and achieve good therapeutic effect and uniform particle size distribution of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] prescription:
[0038] Lecithin 5 grams, cholesterol 1 gram; bufalin 1 gram.
[0039] Preparation Process:
[0040] Take lecithin, cholesterol and bufa fat-soluble extracts such as bufalin and dissolve them in 18ml of chloroform, then remove the chloroform under reduced pressure at a temperature of 40°C, add 100ml of water for injection, treat in an ultrasonic water bath for 15 minutes, homogenize under high pressure, and filter , freeze-dried using conventional methods.
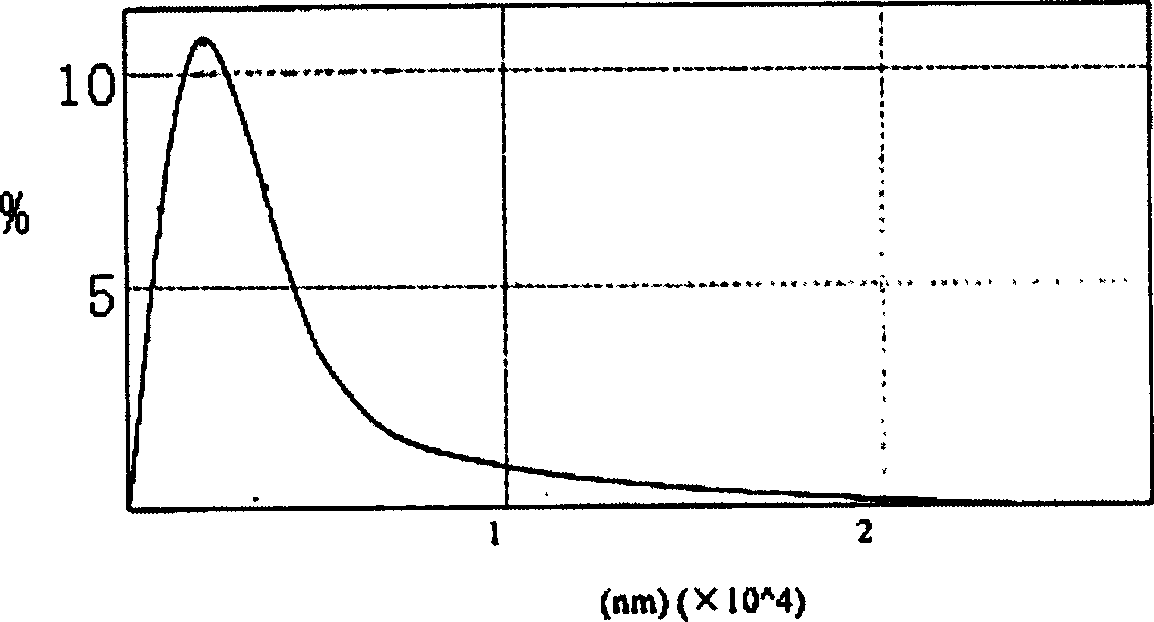
[0041] Its particle size distribution is as figure 1 , figure 1 It is the particle size distribution figure measured by the laser diffraction method, as can be seen from the figure, the average particle size is 80nm, and no particle greater than 200nm is seen.
Embodiment 2
[0043] prescription:
[0044] Lecithin 8 grams, cholesterol 1 gram, toad water-soluble extract 0.5 grams, dextran 10 grams.
[0045] Preparation Process:
[0046] After dissolving lecithin and cholesterol with 15ml ethanol, drop by drop into TongN 2 200ml of bufa lipid aqueous extract and dextran aqueous solution were dissolved with rapid stirring, filtered with 5u, 0.45u, 0.2u filter membranes, and then freeze-dried by conventional methods. The particle size distribution measured by the laser diffraction method is: the average particle size is 80nm, and no particle larger than 500nm is found.
Embodiment 3
[0048] prescription:
[0049] Part (A): 9 grams of lecithin, 2 grams of cholesterol, 1 gram of bufafolin, 0.5 gram of lipobufagenin, and 0.14 gram of cinobufagin.
[0050] Part (B): 5 grams of lecithin, 1 gram of cholesterol, 0.5 grams of toad water-soluble extract, 10 grams of dextran or 20 grams of mannitol.
[0051] Preparation Process:
[0052] (1) Dissolve all the materials in part (A) in chloroform, reduce the pressure on a water bath at 40°C, and remove the chloroform to obtain a drug-containing phospholipid film;
[0053] (2) After dissolving lecithin and cholesterol with ethanol, drop by drop into TongN 2 Rapidly stirred aqueous solution of bufa lipid aqueous extract and dextran aqueous solution or mannitol aqueous solution;
[0054] (3) Add the product of step (1) and step (2) into water, and stir under N 2 Dissolve, homogenize under high pressure, pass through a 0.45u, 0.2u filter membrane, and freeze-dry using a conventional method.
[0055] The average partic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com