Synthesis method of single optical isomer nitrendipine
A synthesis method, photoisomerization technology, applied in the direction of organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
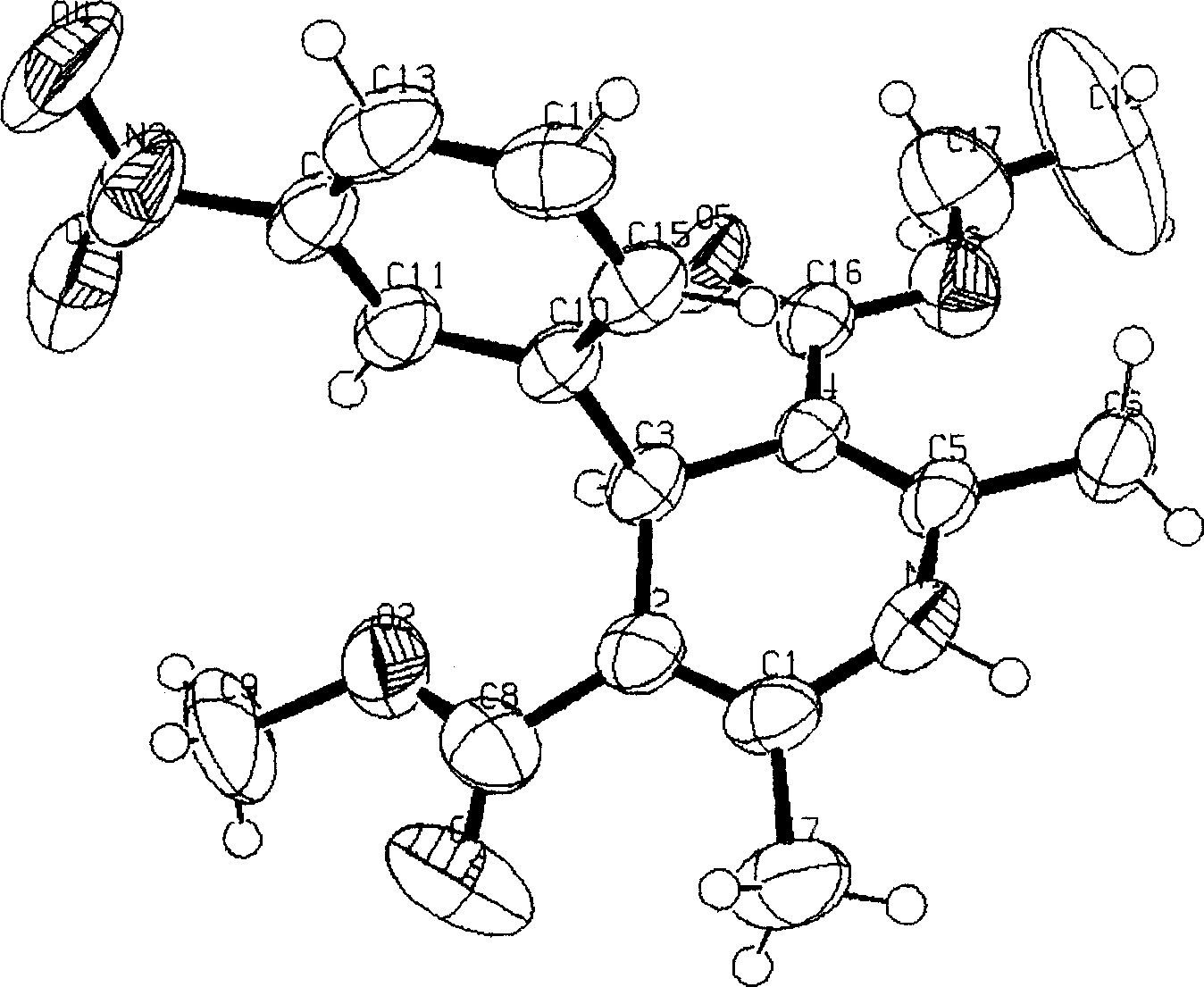
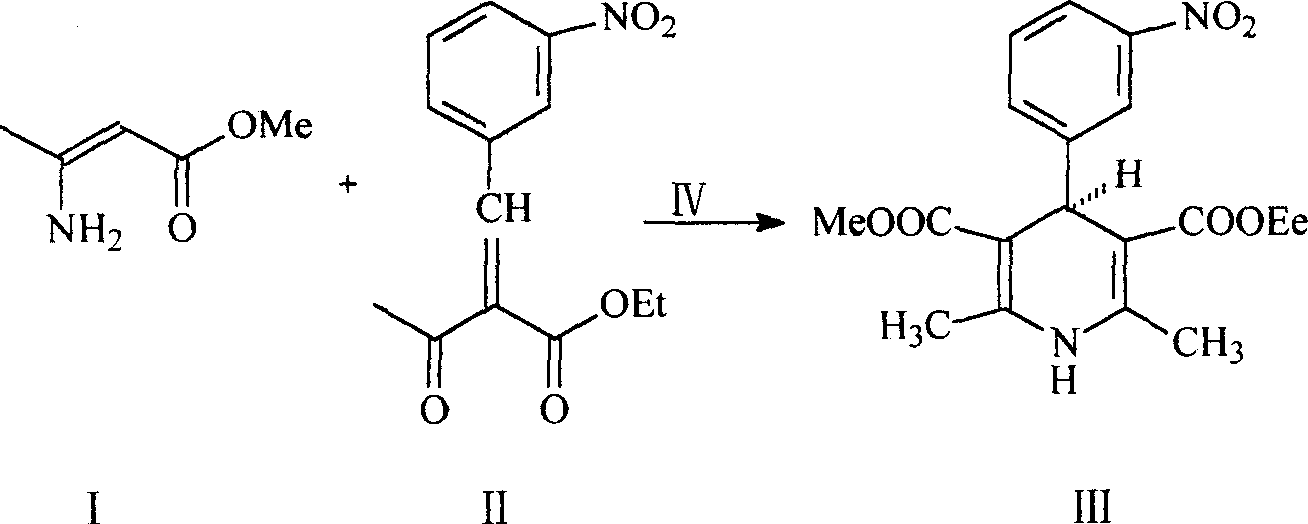
[0008] The synthesis method of this single optical isomer nitrendipine is as follows: using absolute ethanol as a solvent, 2-(3-nitro)benzylidene acetoacetate and β-aminocrotonic acid methyl ester are in the chiral phase. In the presence of the transfer catalyst quinine benzyl ammonium bromide, a stereoselective condensation reaction is performed to obtain the product.
[0009] The reaction formula is as follows:
[0010]
[0011] The specific synthesis example is: add 20ml of absolute ethanol, 13.2g (0.05mol) of ethyl 2-(3-nitrobenzylidene)acetoacetate (II), methyl β-aminocrotonate ( I) 5.75g (0.05mol), 0.13g of quinine benzylammonium bromide (IV), stirred and refluxed for reaction, the temperature was controlled at 78°C, and the reaction time was 4 hours. After the reaction, the reaction solution was distilled under reduced pressure to evaporate part of the ethanol. The reaction solution was frozen for 3 hours and filtered with suction to obtain yellow crystals, which were rec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com