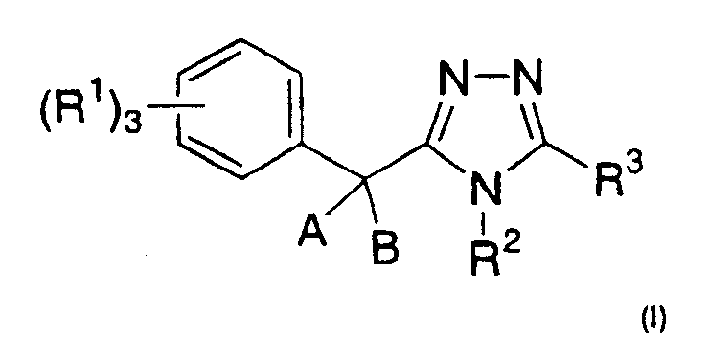
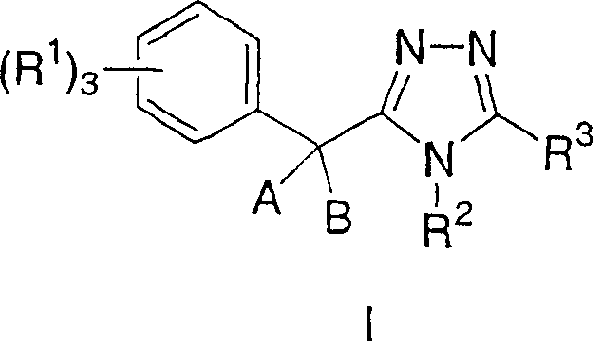
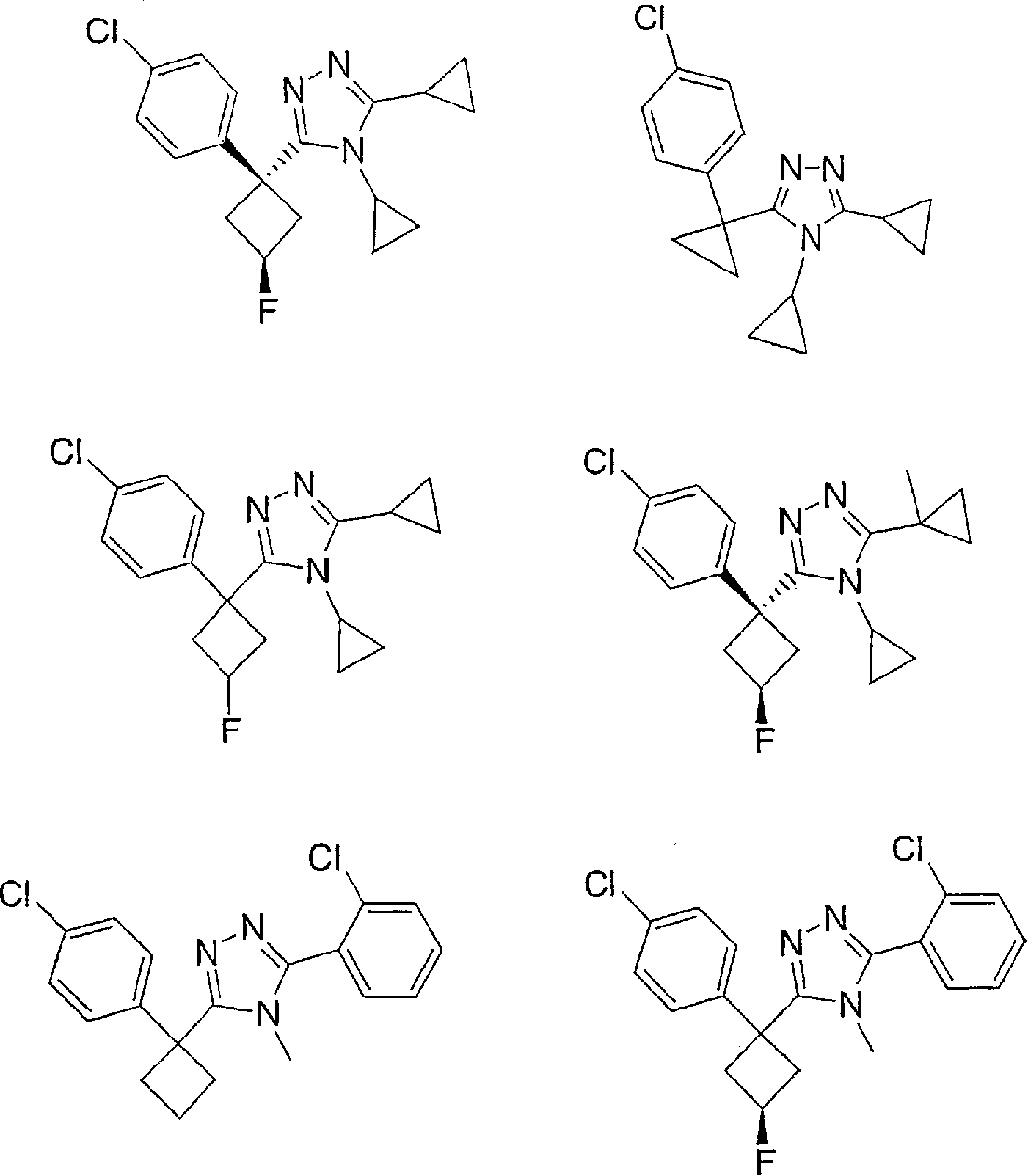
11-beta-hydroxysteroid dehydrogenase 1 inhibitors useful for the treatment of diabetes, obesity and dyslipidemia
A solvate, alkyl technology, applied in the field of enzyme inhibitor of type I 11-β-hydroxysterol dehydrogenase, can solve problems such as lactic acidosis, nausea and diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] general process
[0181]
[0182] substance
quantity
concentration
Mmol
S.M. in DMF
714μl
0.14M in DMF
0.1
TFFH in DMF
200μl
0.5M in DMF
0.1
Triethylamine in DMF
400μL
0.5M in DMF
0.2
Hydrazine in DMF
240μl
0.5M in DMF
0.12
Imino ether A in DMF
600μL
0.25M in DMF
0.15
[0183] The synthesis of the following one-dimensional, single pure compound library was completed in Myriad Core System. All reaction vessels were dried at 120° C. for 12 hours under nitrogen flow before use. Solvents were dried over molecular sieves for at least 12 hours before use. Reagents and subgroups (carboxylic acids and 8-methoxy-2,3,4,5,6,7-azocine (iminoether A)) were dissolved in a suitable solvent immediately before use.
[0184] Synthesis The carboxylic acids shown in the table below were added as starting materials to a dry 10 mL sintered Myria...
Embodiment 2
[0217] Method 2A
[0218] general process
[0219]
[0220] Preparation of 3-(1,1-diphenylpropyl)-5,6,7,8,9,10-hexahydro[1,2,4]triazolo[4,3-a]azocine ( 2-1)
[0221]
[0222] 2,2-Diphenylbutyric acid (39.6mg, 0.166mmol) was dissolved in DMF (0.33ml). Fluoro-N,N,N',N'-tetramethylformamide hexafluorophosphate (TFFH, 43.6 mg) and anhydrous triethylamine (46.4 μl) were added, and the solution was cooled to 0°C. After 10 minutes, hydrazine monohydrate (6.5 μl) was added. After stirring at room temperature for 30 minutes, HPLC / MS showed complete conversion to 2,2-diphenylbutanylhydrazide. 8-Methoxy-2,3,4,5,6,7-hexahydroazocine (38ml, 0.249mmol) was added, and the solution was stirred overnight at 120°C. After warming to room temperature, the product was purified by preparative HPLC and isolated as the trifluoroacetate salt. The salt was added to saturated sodium bicarbonate solution and extracted with ethyl acetate to give the free base. The extract was dried over magne...
Embodiment 3
[0302] Method 3A
[0303] Preparation of 1-(4-chlorophenyl)cyclobutanehydrazide
[0304]
[0305] 1-(4-Chlorophenyl)cyclobutanecarboxylic acid (10.0g) was dissolved in dichloromethane (150ml) and cooled to -10°C in an ice / brine bath. Pyridine (3.84ml) was added followed by cyanuric fluoride (8.9ml in 25ml dichloromethane). After stirring at room temperature for 1 hour, TLC indicated that the reaction was complete. The solution was added to a separatory funnel filled with ice (150 mL). After vigorous shaking, the organic layer was removed, dried over magnesium sulfate, filtered, and concentrated to give carbonyl fluoride.
[0306] Hydrazine (2.02 mL) was dissolved in acetonitrile (100 mL) and cooled to 0°C. Triethylamine (12.8ml) was added followed by 1-(4-chlorophenyl)cyclobutaneoyl fluoride (10g) in acetonitrile (25ml). After stirring at room temperature for 1 hour, the acetonitrile was removed by evaporation. The product was obtained after chromatography on silica g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com