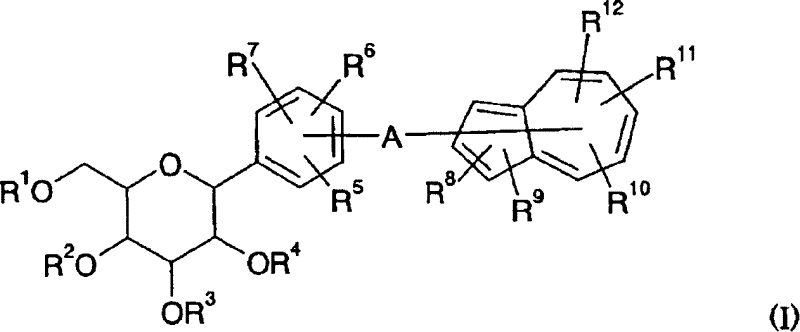
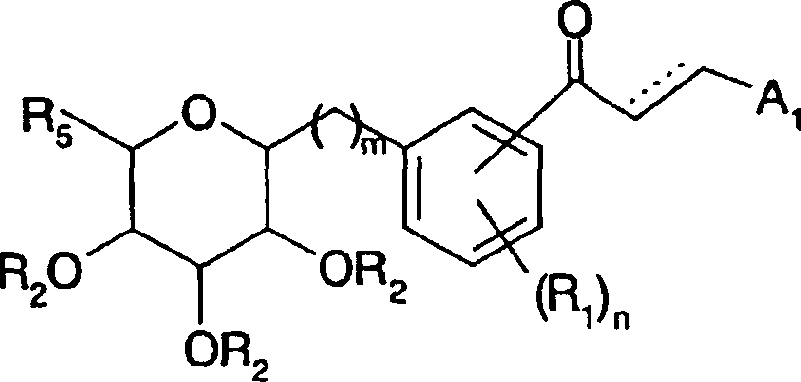
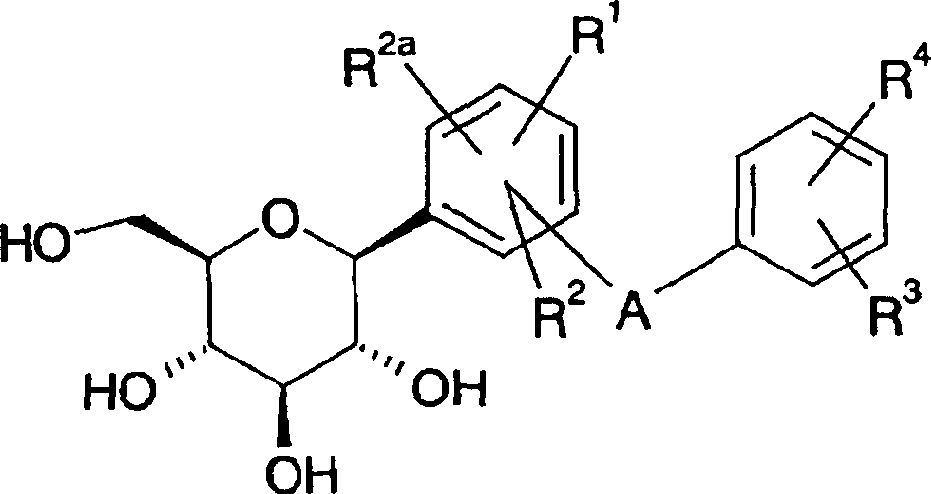
Azulene derivatives and salts thereof
A technology of derivatives and compositions, applied in the field of azulene derivatives and their salts, can solve problems such as the disappearance of hypoglycemic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] At -40°C, boron trifluoride-ether complex (0.39ml) and triisopropylsilane (1.23ml) were added dropwise to 2,3,4,6-tetra-O-benzyl-1-C- In a solution of [3-[(3-methylazulen-1-yl)methyl]phenyl]-D-glucopyranose (2.3 g) in acetonitrile (40 ml), the mixture was stirred for 2 hours. Saturated aqueous potassium carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated. The residue was purified by silica gel column chromatography (n-hexane-ethyl acetate) to obtain (1S)-1,5-anhydro-2,3,4,6-tetra-O-benzyl-1-[3-[(3 -Methylazulen-1-yl)methyl]phenyl]-D-glucitol (1.47 g).
Embodiment 2
[0181] At -78°C, 1M boron tribromide in n-heptane (20ml) was added dropwise to (1S)-1,5-anhydro-2,3,4,6-tetra-O-benzyl-1-[ 3-[(3-Methylazulen-1-yl)methyl]phenyl]-D-glucitol (0.76 g) was dissolved in dichloromethane (20 ml), and the mixture was stirred for 30 minutes. Dichloromethane-toluene (ratio: 2:1, 60ml) was added to the reaction mixture, and methanol (6ml) was further added to the mixture. The reaction mixture was cooled to room temperature and concentrated to half its volume, then methanol (25 ml) was added to the mixture, and concentrated. This operation was repeated three times. The resulting residue was purified by silica gel column chromatography (chloroform-methanol) to obtain (1S)-1,5-anhydro-1-[3-[(3-methylazulen-1-yl)methyl]phenyl ]-D-glucitol (0.068 g).
[0182] In the same manner as in Examples 1 and 2, the compounds of Examples 3 and 4 were obtained, respectively.
Embodiment 5
[0184] In an argon atmosphere, two drops of 1,2-dibromoethane were added dropwise to a suspension of zinc powder (0.17 g) in THF (5.0 ml), and the mixture was heated to reflux for 5 minutes. After cooling to room temperature, two drops of chlorotrimethylsilane were added to the reaction mixture. The mixture was stirred for 15 minutes. Next, (1S)-2,3,4,6-tetra-O-acetyl-1,5-anhydro-1-[5-(bromomethyl)-2-ethoxybenzene was added to the reaction mixture Base]-D-glucitol (1.4 g), the mixture was heated under reflux for 1 hour. After cooling to room temperature, tetrakis(triphenylphosphine)palladium(0) (0.27 g) and methyl 2-chloroazulen-1-carboxylate (0.28 g) were added to the reaction mixture. The mixture was heated to reflux for 6 hours. After cooling to room temperature, the reaction mixture was poured into 10% aqueous hydrochloric acid under ice cooling. The mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine and dried over anhydrous so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com