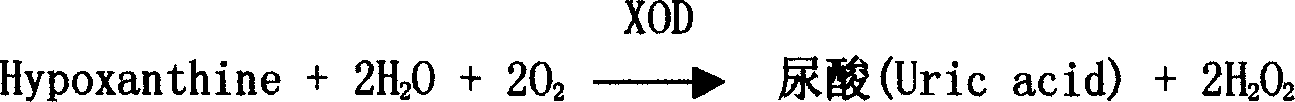Method and kit for investigating 5'-nucleotidase by coupling enzymatic reaction
A nucleotidase and inosinic acid technology, which is applied in biological testing, microbial measurement/inspection, biochemical equipment and methods, etc., can solve the problem of narrow selection of hydrogen donors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] 1. Determine the IMP Michaelis constant Km
[0029] 1) Reagent composition
[0030] Reagent I Good's buffer pH 7.6 100mmol / L
[0031] 4-AA 2mmol / L
[0032] 5’-NT 150U / L (rattlesnake venom)
[0033] PNP 100U / L
[0034] XOD 200U / L
[0035] POD 600U / L
[0036] Reagent II Good's buffer pH 7.6 100mmol / L
[0037] IMP 0.001-10mmol / L
[0038] TOOS 2mmol / L
[0039] 2) Test parameters and operation steps
[0040] Temperature 37°C
[0041] Wavelength 550nm
[0042] Cuvette light path 1.0cm
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com