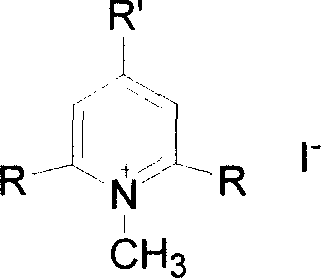
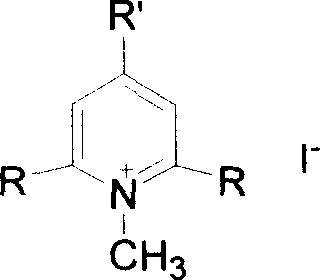
Six kinds of compound of polysubstituted pyridinium and preparation method
A pyridinium salt compound, pyridinium salt technology, applied in chemical instruments and methods, organic chemistry, metallocene and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: Preparation of iodide N-methyl-2,4,6-tris[(E)-2-(4-ferrocenylphenyl)vinyl]pyridinium salt
[0015] With 13.2 parts by weight of iodide N-methyl-2,4,6-collidine salt, 87 parts by weight of p-4-ferrocenyl benzaldehyde are dissolved in 237 parts by weight of methanol and 148 parts by weight of tris In a mixed solvent of methyl chloride, stir, heat up to 80° C., reflux for 8 hours, slowly cool, and precipitate a solid, silica gel column chromatography, the eluent is dichloromethane:methanol (volume ratio)=50:1, and 22.1 parts are obtained The corresponding trisubstituted pyridinium iodide N-methyl-2,4,6-tris[(E)-2-(4-ferrocenylphenyl)vinyl]pyridinium salt yielded 41%.
Embodiment 2
[0016] Example 2: Preparation of N-methyl-2,6-bis[(E)-2-(4-ferrocenylphenyl)vinyl]pyridinium iodide
[0017] 12.5 parts by weight of iodide N-methyl-2,6-lutidine salt, 43.5 parts by weight of p-4-ferrocenyl benzaldehyde were dissolved in 158 parts by weight of methanol, stirred, refluxed for 4 hours, slowly Cooling, solid is separated out, the solid that is separated out is recrystallized twice in methanol, each time with the methyl alcohol of 474 parts by weight, obtains the corresponding disubstituted pyridinium iodide N-methyl-2 of 29.4 parts by weight, 6-bis[ (E)-2-(4-ferrocenylphenyl)vinyl]pyridinium salt, yield 74%.
Embodiment 3
[0018] Example 3: Preparation of iodide N-methyl-2,4,6-tris[(1E,3E)-4-ferrocenyl-1,3-butadienyl]pyridinium salt
[0019] 13.2 parts by weight of N-methyl-2,4,6-collidine iodide, 72.0 parts by weight of ferrocene acrolein are dissolved in a mixture of 237 parts by weight of methanol and 148 parts by weight of chloroform In solvent, stirred, reacted at room temperature for 48 hours, precipitated solid, the precipitated solid was subjected to silica gel column chromatography, and the eluent was dichloromethane:methanol (volume ratio)=50:1 to obtain 15.8 parts by weight of the corresponding trisubstituted Pyridinium iodide N-methyl-2,4,6-tris[(1E,3E)-4-ferrocenyl-1,3-butadienyl]pyridinium salt with a yield of 34%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com