AFP-antigen-based AFP nanobody A80
A nano-antibody and antigen technology, applied in the biological field, can solve the problems of expensive antibody and antigen standard products, and achieve the effects of small molecular weight, good specificity and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
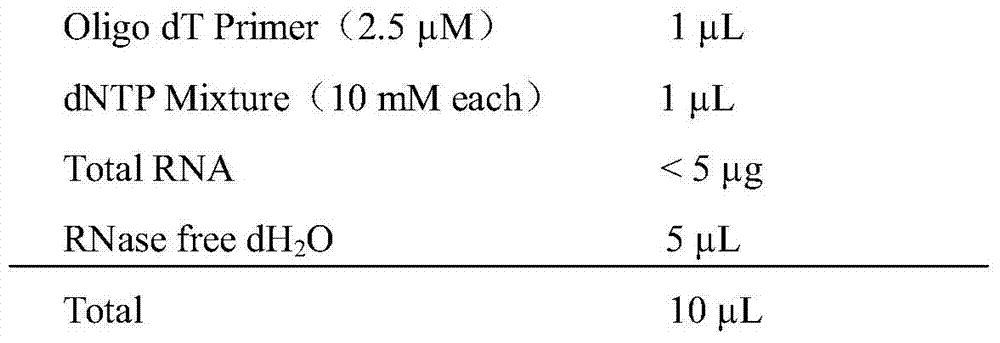
[0022] Example 1. Construction of camel-derived immune single-domain heavy chain antibody library
[0023] 1) Immunity of alpaca:
[0024] A healthy adult alpaca was selected and immunized by intradermal and subcutaneous injections on the back. The primary immunization was Freund's complete adjuvant, and the immunogen dose was 1 mg; the second immunization on the 28th day was Freund's incomplete adjuvant. The immunogen dose was 0.5 mg, the third dose on the 49th day, Freund's incomplete adjuvant, and the immunogen dose was 0.25 mg; the fourth dose on the 70th day, Freund's incomplete adjuvant, the immunogen dose was 0.25 mg. On the seventh day after the third booster immunization, alpaca peripheral blood was collected for the construction of a phage display library.
[0025] 2) Separation of camel-derived leukocytes: add lymphocyte separation solution to the centrifuge tube, then add blood samples, centrifuge at 700g for 20min; carefully suck the leukocytes suspended in the m...
Embodiment 2
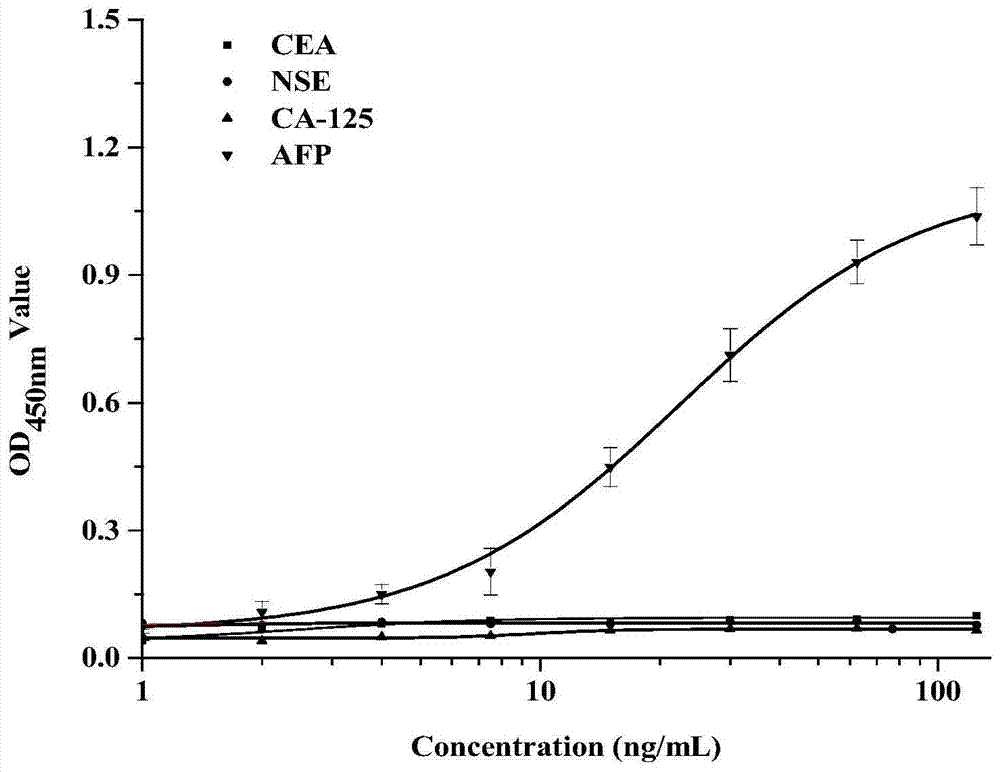
[0049] Example 2. Affinity panning and identification of AFP Nanobodies
[0050] 1) Biotinylation of AFP as a target molecule
[0051] A Dissolve 2 mg of target protein in 1 mL of 50 mM NaHCO 3 (pH 8.5);
[0052] B Before use, dissolve 1 mg of Sulfo-NHS-LC-biotin in 1 mL of water, shake and mix well. Add 74 μL of this solution to the target protein solution;
[0053] C Put the tube on ice for 2h;
[0054] D Dialyze to remove unreacted biotin, dialyze against 1 liter of TBS buffer, and replace the dialysate at least twice;
[0055] E Quantify biotinylated protein with Nano Drop.
[0056] 2) Panning
[0057] with streptavidin (100 μg / mL streptavidin in 0.1 M NaHCO 3 (pH 8.6)) coated microtiter plate, 37 ℃ 2h. After washing 3 times with PBST (10mM PBS, 0.5% Tween-20 (v / v)), add 300 μL of 3% OVA-PBS and block for 1 hour at 37°C (blocking solution should contain 0.1 μg / mL streptavidin). In order to bind the biotin contained in the BSA blocking solution), the phage was prev...
Embodiment 3
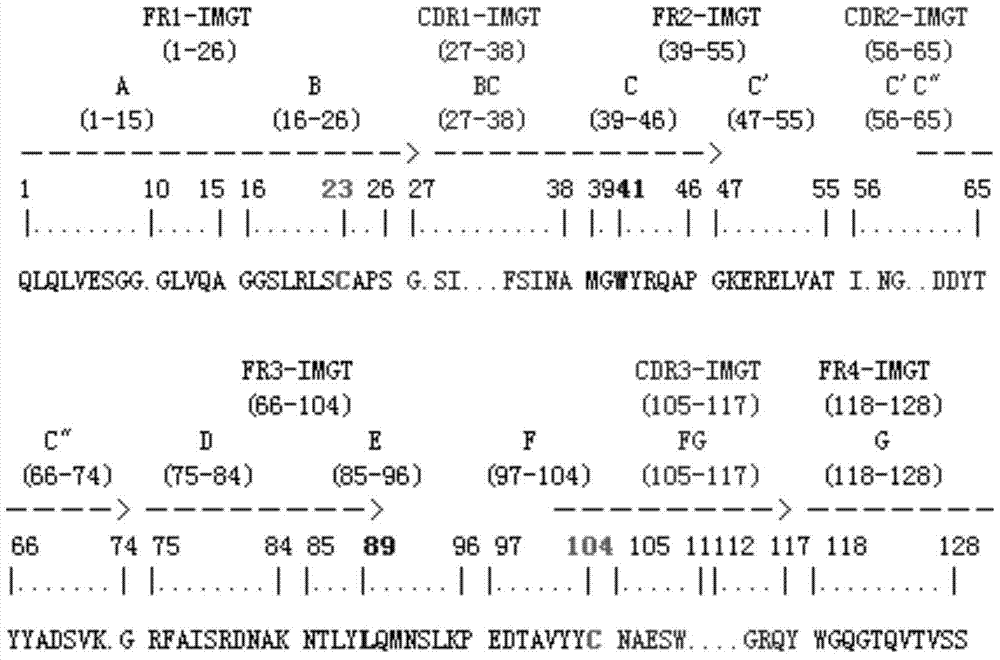
[0068] Example 3. Sequencing of AFP Nanobody Encoding Gene and Determination of Amino Acid Sequence
[0069] The positive phage clones identified by the double-antibody sandwich enzyme-linked immunosorbent assay method were subjected to DNA sequencing, and the amino acid sequence was obtained according to the DNA sequencing results and the codon table.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com