Expression purification in colibacillus and activity identification method of recombinant thymulin alpha-1
A technology for expression and purification of Escherichia coli, which is applied in the field of medical bioengineering, can solve problems such as low yield, high production cost, and cumbersome steps, and achieve the effects of reducing production cost, shortening cycle, and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
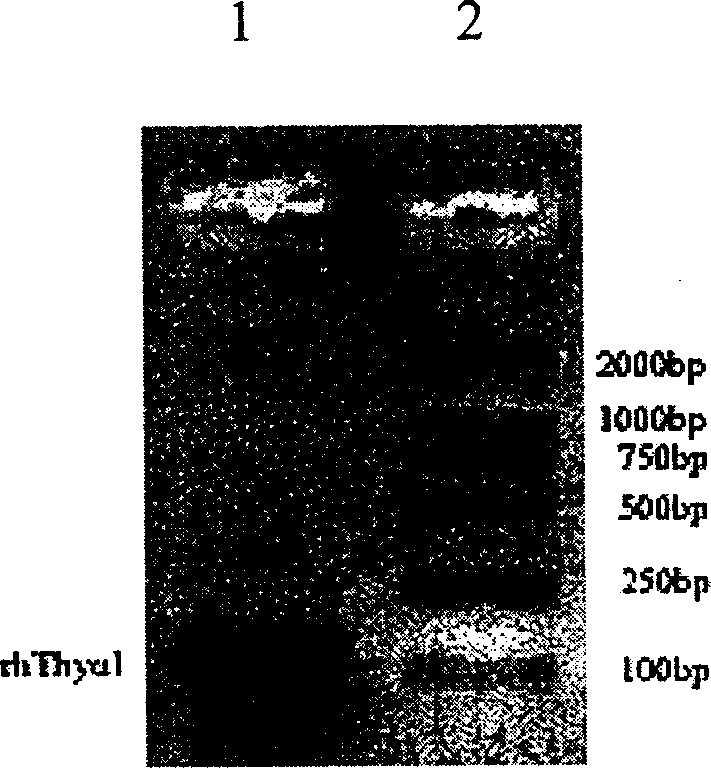
[0038] 1. Take 1 μL chemically synthesized rhThyα for PCR reaction 1 Template (5nmol / L) was added to 36.75μL sterile water, followed by 50pmol 5' end primer and 3' end primer, 5μL 10×PCR buffer, 1μL 10mmol / L dNTP, 4μL 25mmol / L MgCl 2 and 0.25 μL Taq enzyme, and amplify immediately after mixing. The reaction conditions were denaturation at 94°C for 1 min; then the following 26 cycles were performed: 94°C for 45 s, 54°C for 1 min, 72°C for 45 s; and extension at 72°C for 8 min.
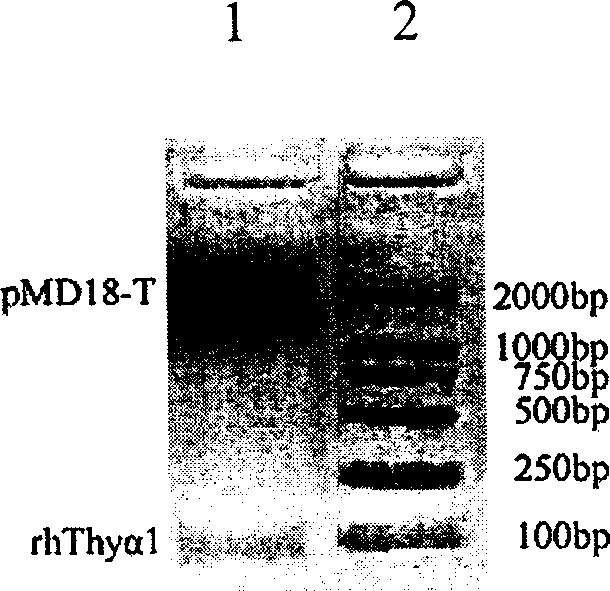
[0039] 2. rhThyα 1 Cloning plasmid (Thyα 1 Construction of / pMD18-T Vector) Take 4 μL of PCR amplification product, mix with 5 μL of ligation buffer and 1 μL of ligation vector (containing ligase), and incubate at 16° C. for 30 min. Take 5 μL of the ligation product to transform into DH5α competent cells, use KpnI / SacI double enzyme digestion to screen and identify positive clones, and perform sequence determination.
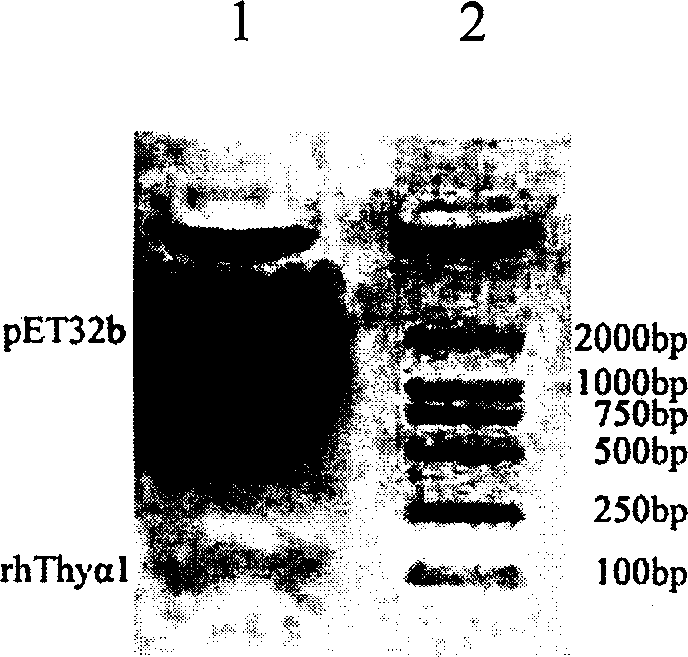
[0040] 3. rhThyα 1 Expression plasmid (Thyα1 / pET32b) construction Thyα 1 The / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com