Diaryl neptanone compound and its preparing method
A technology for diarylheptanone and compound, which is applied in the field of new diarylheptanone compound and its preparation, and can solve problems such as undiscovered new type compound.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
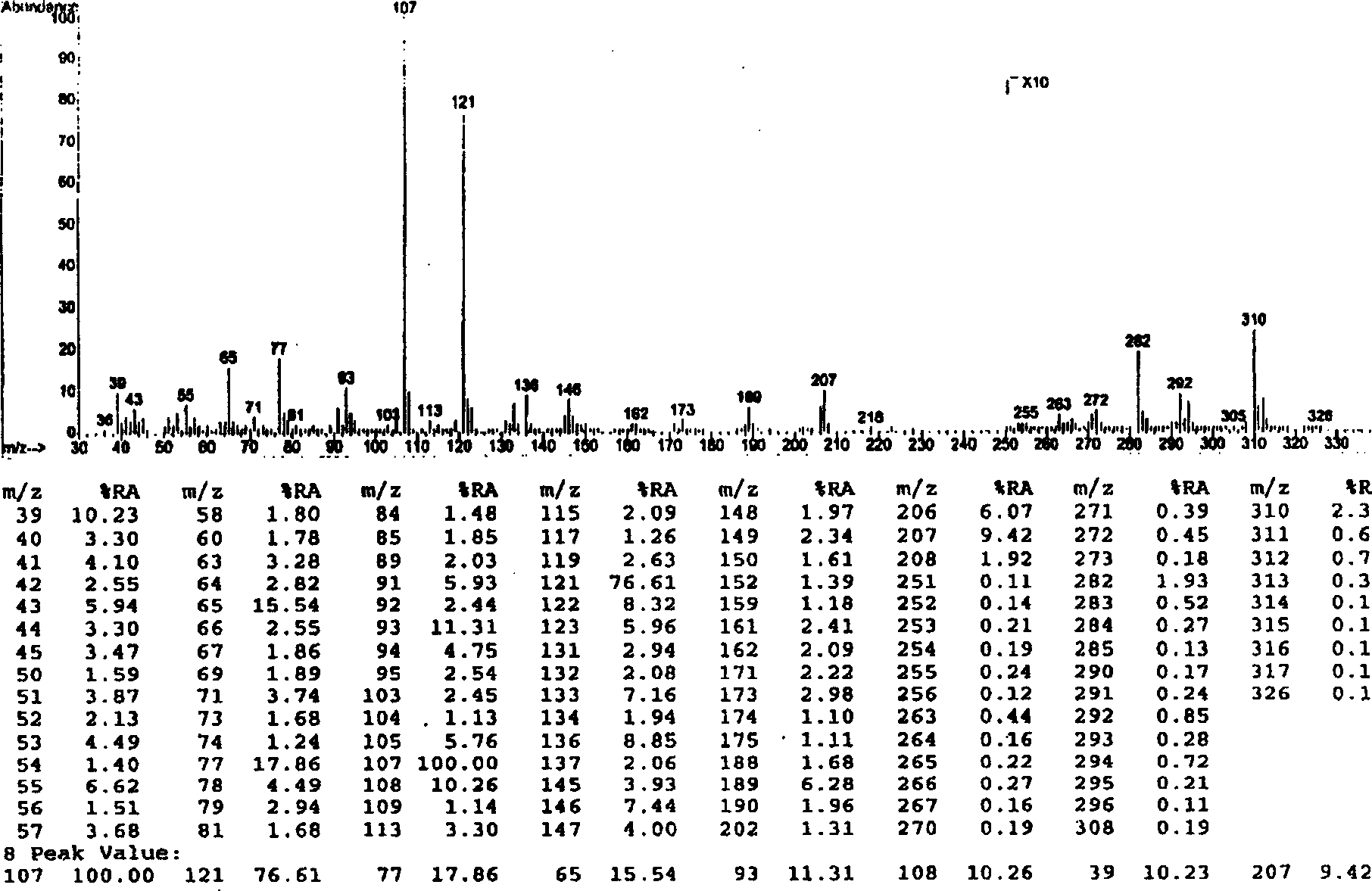
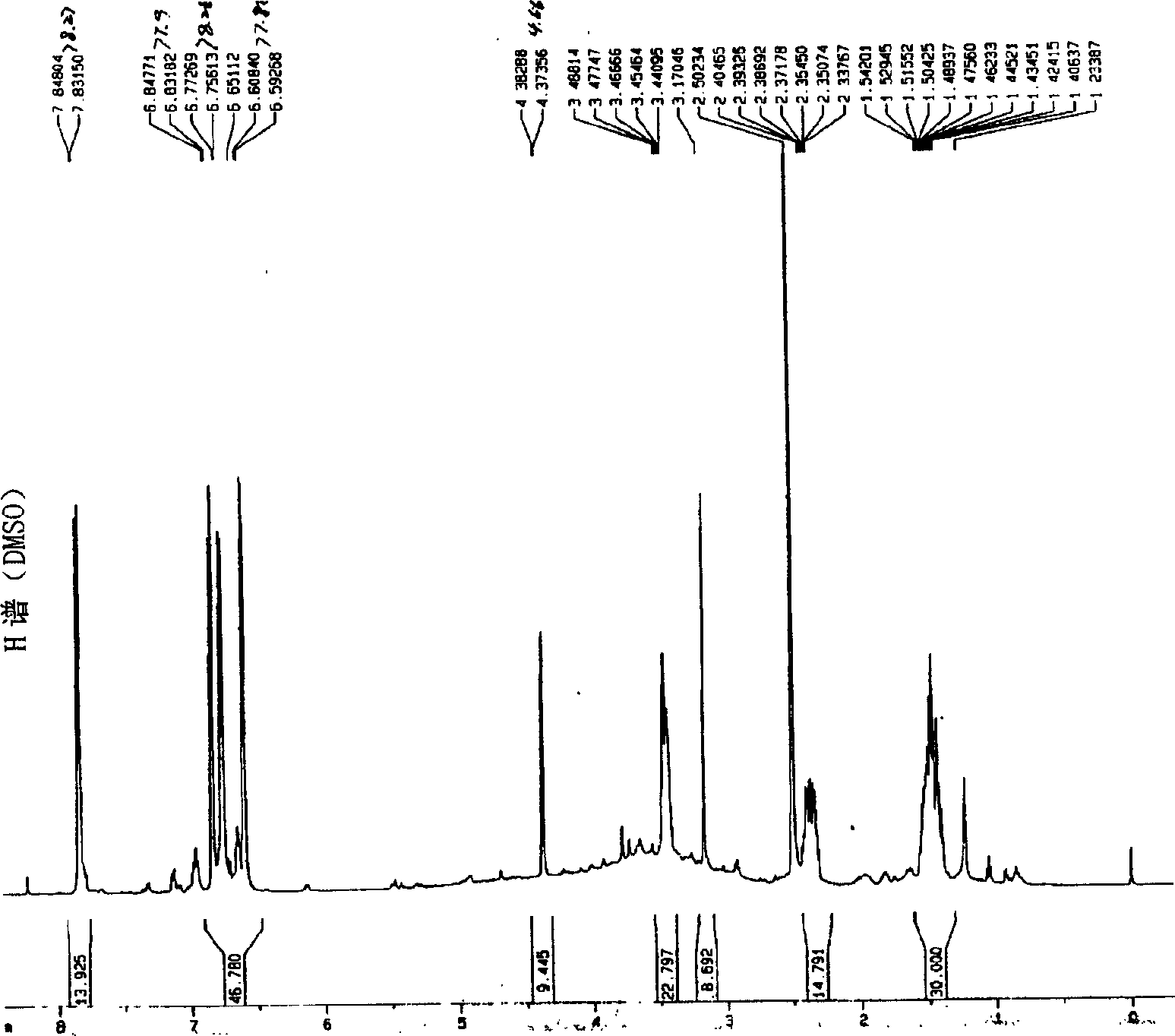
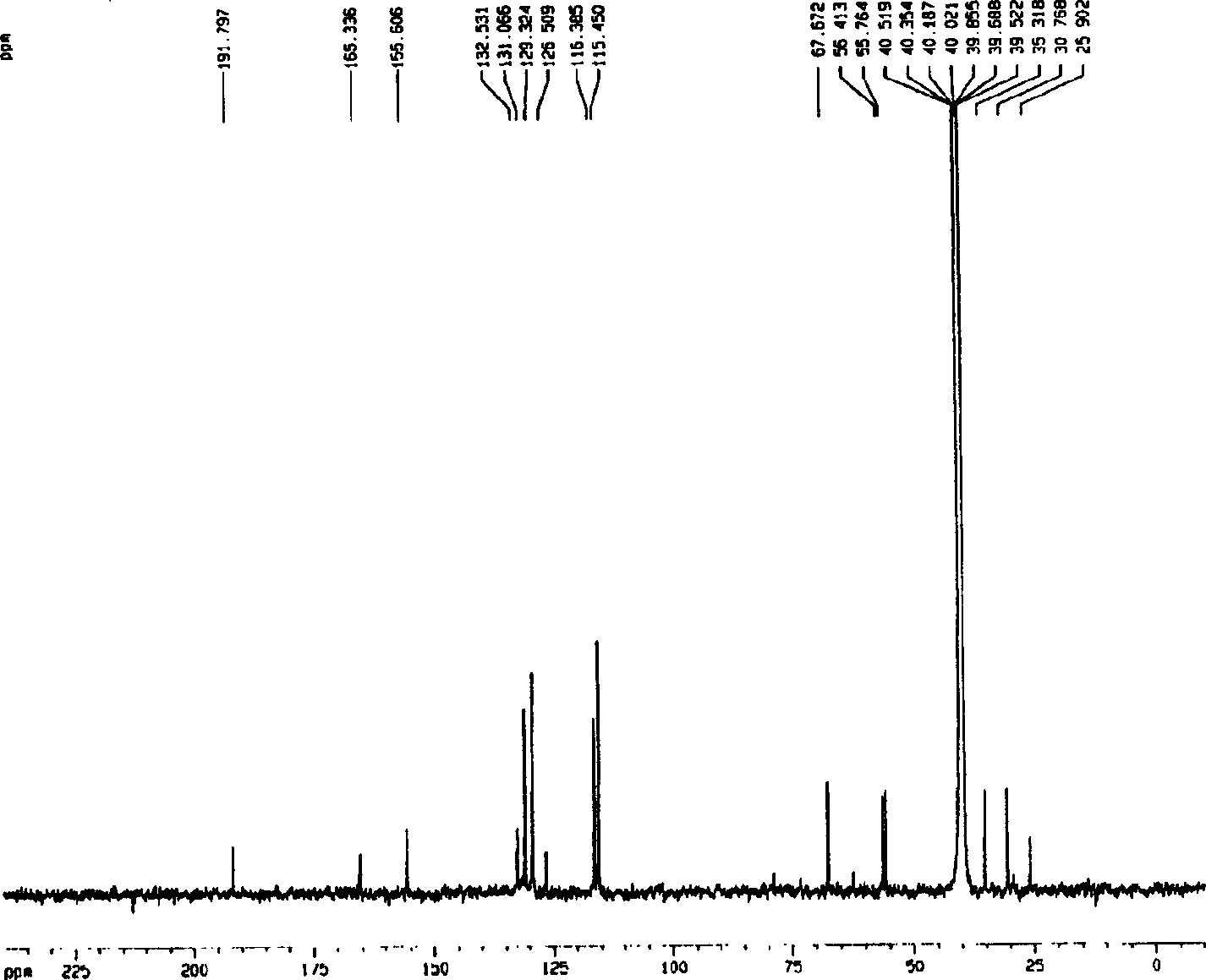
[0051] Embodiment 1.1, the separation and preparation of 7-bis(4'-hydroxyphenyl)-5-hydroxyl-(2,3) epoxy-1-heptanone
[0052]Plant material: Dried stems and branches of mistletoe (Viscus coloratum (Kom.) Nakai) of the genus Mistletoe of the Morusaceae family.
[0053] Separation and preparation method: 2.0kg of stems and branches of mistletoe with leaves, reflux extraction twice with 20L of 95% ethanol, each time for 2 hours, combine the extracts, recover the ethanol and concentrate until it has no alcohol smell, add water to adjust to 4.0L, and filter with suction , the clarified liquid passes through D 101 The macroporous resin column (1.0Kg) was eluted with 4-5L of water, 20%, 40%, 75% and 95% ethanol in sequence, and 40% of the eluted fraction was collected and concentrated to dryness. The fraction eluted with 40% ethanol was repeatedly separated with Sephadex LH 20 to obtain 1,7-bis(4'-hydroxyphenyl)-5-hydroxy-(2,3)epoxy-1-heptanone. The extraction and separation flow ch...
Embodiment 2
[0053] Separation and preparation method: 2.0kg of stems and branches of mistletoe with leaves, reflux extraction twice with 20L of 95% ethanol, each time for 2 hours, combine the extracts, recover the ethanol and concentrate until it has no alcohol smell, add water to adjust to 4.0L, and filter with suction , the clarified liquid passes through D 101 The macroporous resin column (1.0Kg) was eluted with 4-5L of water, 20%, 40%, 75% and 95% ethanol in sequence, and 40% of the eluted fraction was collected and concentrated to dryness. The fraction eluted with 40% ethanol was repeatedly separated with Sephadex LH 20 to obtain 1,7-bis(4'-hydroxyphenyl)-5-hydroxy-(2,3)epoxy-1-heptanone. The extraction and separation flow chart is attached Figure 4 . Embodiment 2.1, the scavenging effect of 7-two (4'-hydroxyphenyl)-5-hydroxyl-(2,3) epoxy-1-heptanone to hydroxyl radical and superoxide anion radical
[0054] 1. Instruments and materials
[0055] 1.1 Instruments:
[0056] UV-260 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com