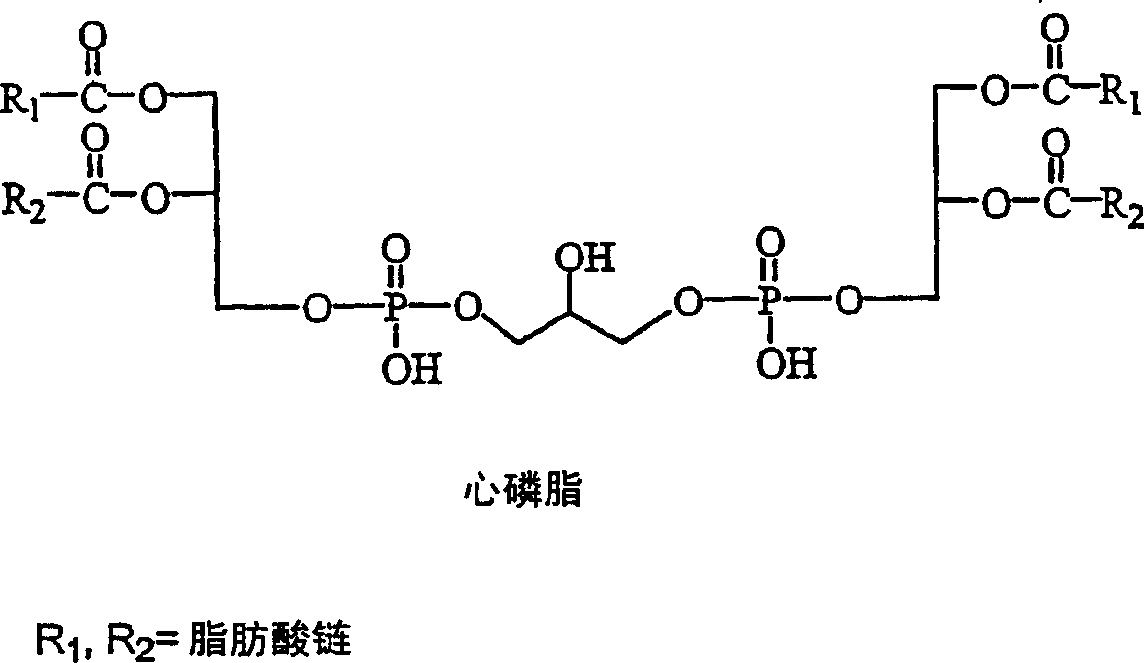
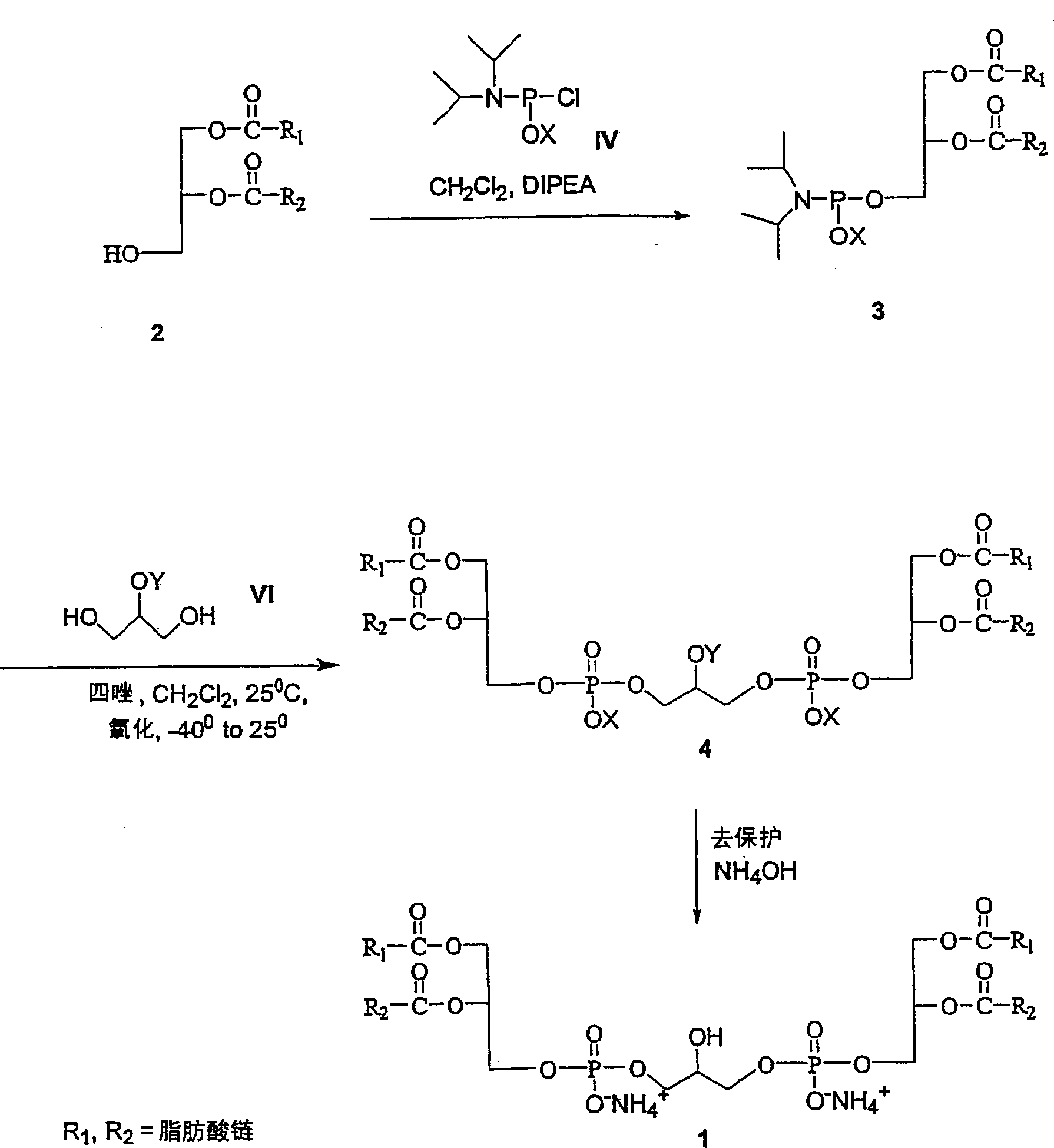
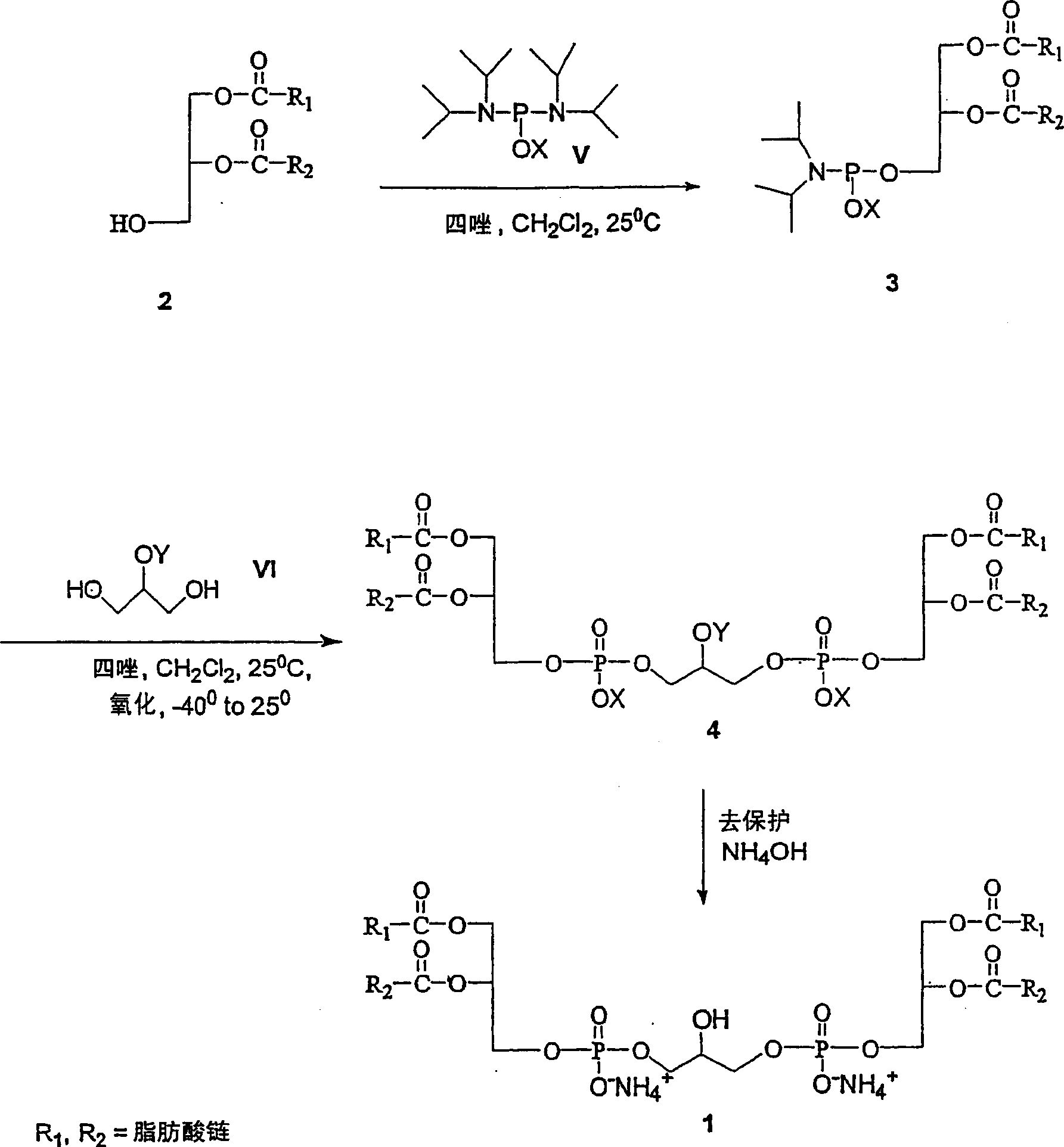
Cardiolipin molecules and method of synthesis
A technology of cardiolipin and phospholipid, applied in the field of cardiolipin molecule and synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
[0071] The synthesis of embodiment 1 tetramyristoyl cardiolipin
[0072] 1A. 2-Benzyl-1,3-bis[(1,2-dimyristoyl-sn-propanetriyl-3)-phosphoryl]glycerol dibenzyl ester
[0073]
[0074] R 1 , R 2 = myristoyl (C 14:0 chain)
[0075] 1,2-Dimyristoyl-sn-glycerol (10 g, 19.53 mmol), benzyl N,N-tetraisopropylphosphoramidite (9.87 g, 29.29 mmol) and 1H - Tetrazole (65mL 0.45M acetonitrile sol, 29.29mmol) in CH 2 Cl 2 (125 mL) was stirred for 3 hours. Add 2-benzyloxy 1,3-propanediol (1.18 g, 6.47 mmol) in CH 2 Cl 2 (20 mL) followed by 1H-tetrazole (37.7 mL of 0.45M acetonitrile sol, 16.85 mmol) and stirred for 3 hours. The reaction mixture was cooled to -40°C, and tert-butyl hydroperoxide (TBHP, 6.4 mL of 5-6M decane sol, 32.35 mmol) was added. After stirring at -40 °C for 30 min, the reaction mixture was warmed to room temperature and washed with CH 2 Cl 2 (250mL) diluted, washed {saturated Na 2 SO 3 Aqueous solution (2×50 mL), saturated NaHCO 3 Aqueous...
Embodiment 2 4
[0080] The synthesis of embodiment 2 tetralauroyl cardiolipin
[0081] 2A. 2-Benzyl-1,3-bis[(1,2-dilauroyl-sn-propanetriyl-3)-phosphoryl]glycerol dibenzyl ester
[0082]
[0083] R 1 ,R 2 = Lauroyl (C 12:0 chain)
[0084] Method 1: 1,2-dilauroyl-sn-glycerol (2.2g, 4.82mmol), benzyl N,N-tetraisopropyl phosphoramidite (1.95g, 5.78mmol) and 1H-tetrazole (12.84 mL 0.45M acetonitrile sol, 5.78mmol) in CH 2 Cl 2 (25 mL) was stirred at room temperature under argon for 3 hours. Add 2-benzyloxy 1,3-propanediol (352 mg, 1.92 mmol) in CH 2 Cl 2 (10 mL) followed by 1H-tetrazole (12.84 mL of 0.45M acetonitrile sol, 5.78 mmol) and stirred for 3 hours. The reaction mixture was cooled to -40°C, and 3-chloroperoxyperbenzoic acid (m-CPBA, 2.77 g, 9.64 mmol) was added in portions. After stirring at -40 °C for 30 min, the reaction mixture was warmed to room temperature and washed with CH 2 Cl 2 (150mL) diluted, washed {saturated Na 2 SO 3 Aqueous solution (2×50 mL)...
Embodiment 3 4
[0090] The synthesis of embodiment 3 tetralauroyl cardiolipin
[0091] In this method, tetralauroyl cardiolipin was synthesized via 2-cyanoethylphosphoramidite.
[0092] 3A. 2-Benzyl-1,3-bis[(1,2-dilauroyl-sn-propanetriyl-3)-phosphoryl]glycerol dicyanoethyl ester
[0093]
[0094] R 1 ,R 2 = Lauroyl (C 12:0 chain)
[0095] 1,2-Dilauroyl-sn-glycerol (1.74 g, 3.79 mmol) and N,N-diisopropylethylamine (545 mg, 4.22 mmol) in anhydrous ether (20 mL) were dissolved under argon atmosphere To the mixture of 2-cyanoethyldiisopropylchlorophosphoramidite (1 g, 4.22 mmol) was added. The mixture was stirred at room temperature for 1 hour, the separated diisopropylamine hydrochloride was filtered and the filtrate was concentrated in vacuo. The residue was thus used in the phosphorylation reaction.
[0096] Phosphoramidite and 1H-tetrazole (9.4mL 0.45M acetonitrile sol, 4.22mmol) in anhydrous CH 2 Cl 2 (30 mL), add 2-benzyloxy 1,3-propanediol (312 mg, 1.71 mmol) in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com