Natural and synthetic isothiocyanate kind compound and its application in treating and preventing cancer
A technology of ester compounds and isothiocyanate, which is used in medical preparations containing active ingredients, gene therapy, ester active ingredients, etc. Clinical use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
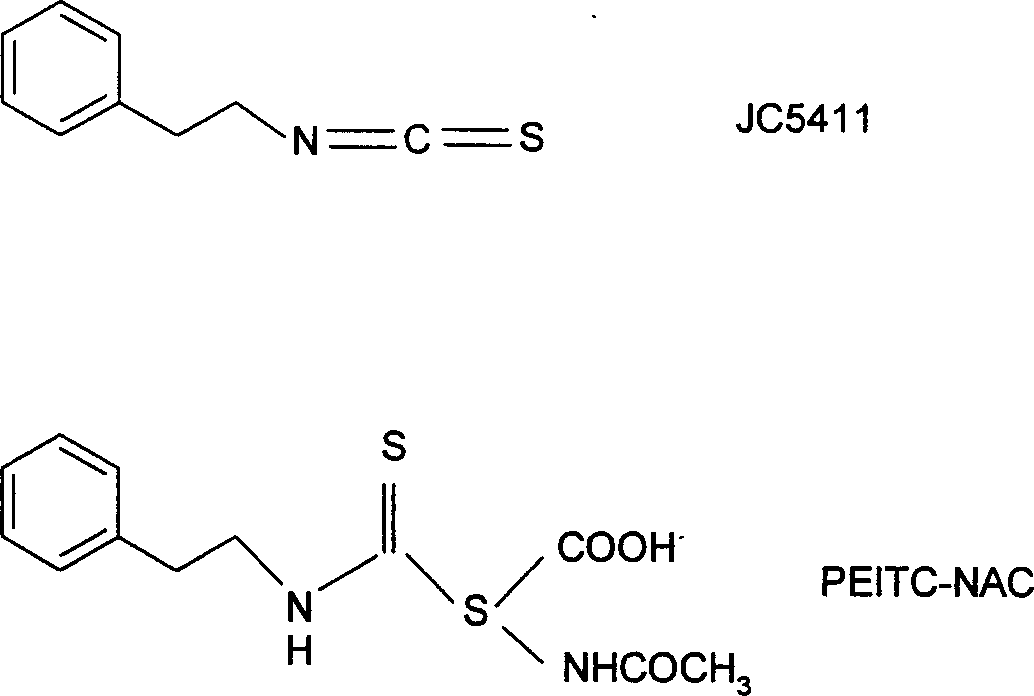
[0062] Example 1, the synthesis of JC-5411
[0063] Instruments and reagents
[0064] 1 The H-NMR spectrum was measured with a Brucker AV-300 nuclear magnetic resonance instrument, the internal standard TMS, and the solvent was CDCl 3 ; MS was determined with a Nicolet FTMS-2000 mass spectrometer; elemental analysis was determined with an Elementar Vario EL III instrument.
[0065] Thin-plate chromatography (TLC) was self-made with silica gel GF254 (produced by Qingdao Ocean Chemical Factory); all reagents were commercially available chemically pure or analytically pure products, and were used directly without treatment.
[0066] Preparation of JC-5411 (Phenethyl Isothiocyanate, Phenethyl Isothiocyanate)
[0067] Into a 50 mL round bottom flask was added 15 mL of CH 2 Cl 2 and 3 mL (40 mmol) of thiophosgene, stirred, cooled to 0 ° C, and slowly added dropwise an equivalent amount of triethylamine (4.04 g, 40 mmol) and phenethylamine (4.85 g, 40 mmol) with a constant pre...
example 2
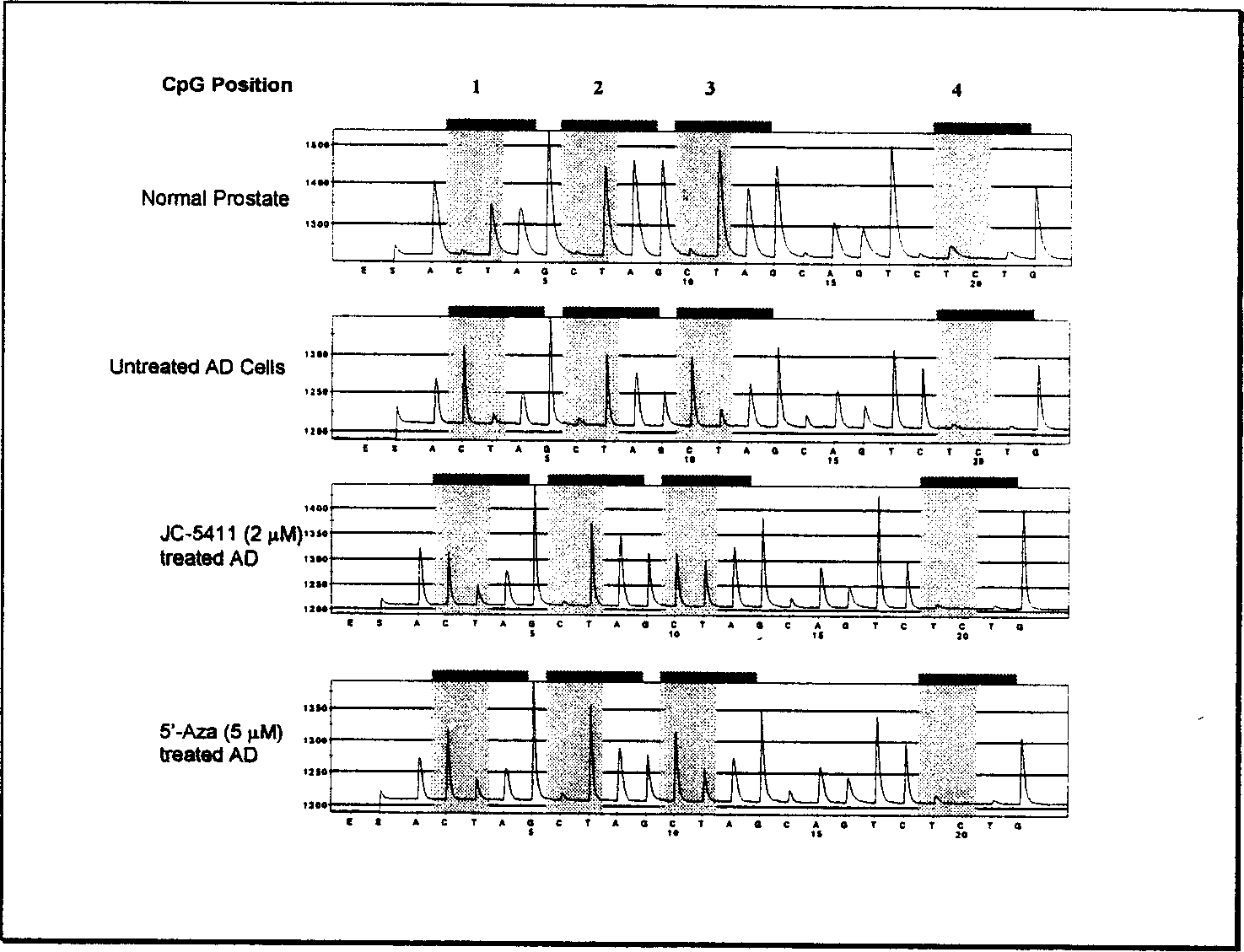
[0069] Example 2, JC-5411 inhibits GSTP1 gene promoter DNA methylation:
[0070] Materials and methods:
[0071] Reagent: JC-5411 Phenethyl Isothiocyanate, synthesized by Wuxi Jesse Pharmaceutical Technology Co., Ltd. (see Example 1). During the experiment, the solution was prepared in DMSO. DNA polymerase chain reaction (PCR) reagents were purchased from Invitrogen Biotechnology Company, USA. PCR primers were purchased from Operon Biotechnology Company in the United States. Other chemical reagents used in the experiment were purchased from Sigma Chemical Reagent Company in the United States unless otherwise specified. Polyacrylamide gel reagents for protein electrophoresis, SDS, electrophoresis buffer, and protein electrotransfer buffer were purchased from Bio-Rad Life Sciences, USA.
[0072] Cell culture: Human prostate cancer cells LNCAP and PC-3 were purchased from American Type Culture Collection. Place cells at 37°C, 5% CO 2 In an incubator, culture in RPMI1640 medi...
example 3
[0080] Example 3, JC-5411 inhibits histone deacetylase:
[0081] Materials and methods:
[0082] Reagent: JC-5411, same as Example 2. Polyacrylamide gel reagents for protein electrophoresis, SDS, electrophoresis buffer, and protein electrotransfer buffer were purchased from Bio-Rad Life Sciences, USA. Site-specific acetylated and methylated histone H3 antibody was purchased from Upstate Biotechnology Company, USA. Unless otherwise specified, other chemical reagents used in the experiment were purchased from Sigma Chemical Reagent Company in the United States. Histone deacetylase (HDAC) activity assay kit was purchased from American Biomol Biochemical Technology Company.
[0083] Cell culture: with example 2.
[0084] Determination of histone acetylation and methylation: Human prostate cancer cells LNCAP in the logarithmic growth phase were treated with different concentrations of JC-5411 for 48 hours, and histones were separated by the method described by Yoshida et al. (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com