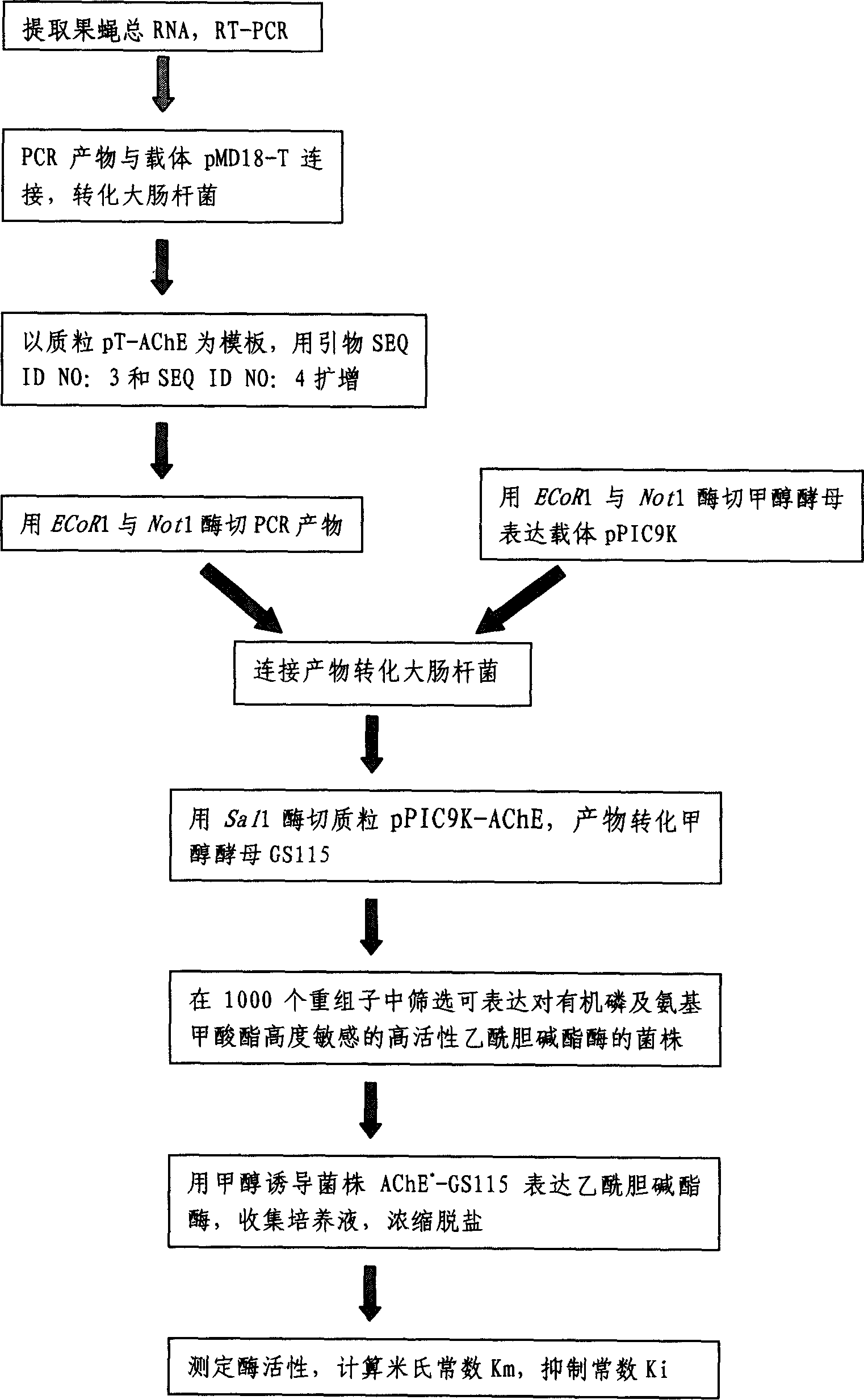
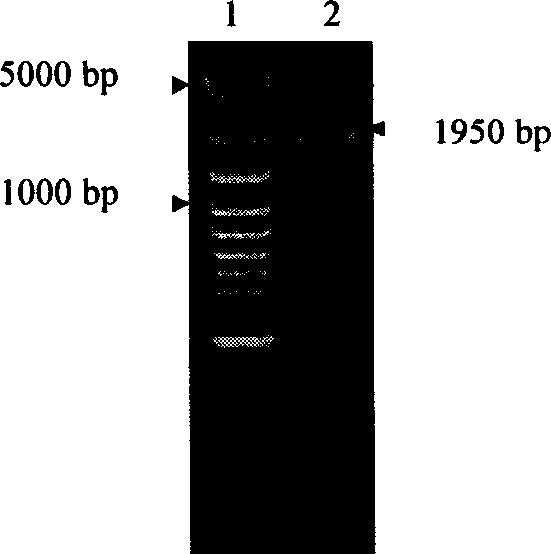
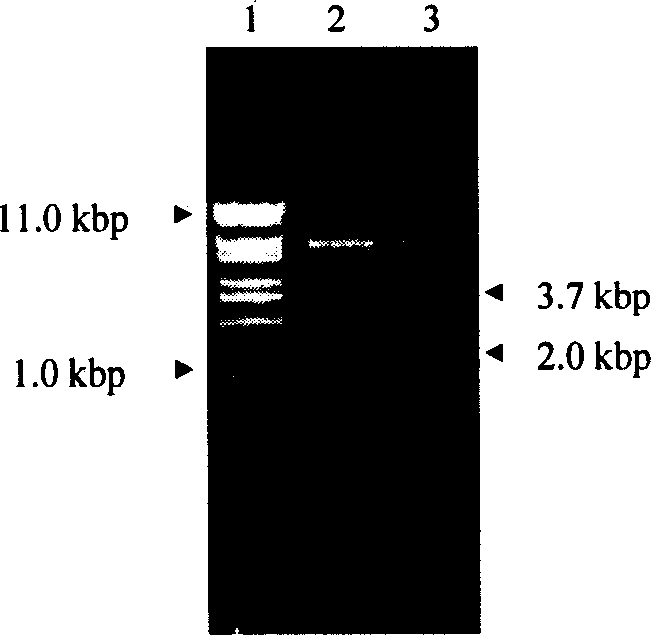
Encoded gene sequence of nucleotide of acetylcholine esterase in fruit fly
A technology of acetylcholinesterase and nucleotide genes, applied in genetic engineering, plant gene improvement, hydrolytic enzymes, etc., can solve undiscovered problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1——The activity of the recombinant AChE protein expressed by yeast is inhibited by a small amount of dichlorvos
[0090] 1. Solutions and reagents
[0091] (1) BMGY solution
[0092] Dissolve 10g of yeast extract and 20g of peptone in 700ml of water, and autoclave (20min); after the temperature of the solution drops to room temperature, add 100ml of potassium phosphate buffer (1M, pH6.0), 100ml of 10×YNB, 2ml of 500×B, 100ml 10×GY; the shelf life of the solution at 4°C is two months.
[0093] (2) BMMY solution
[0094] Change the 10×GY in the BMGY solution to 10×M, and keep the other components unchanged. The solution has a shelf life of two months at 4°C.
[0095] (3) Dichlorvos solution
[0096] 0.11 g of dichlorvos (MW=220.98) crystals were weighed and dissolved in 5 ml of acetone. At this time, the concentration of dichlorvos was 0.1M. Dilute the solution stepwise by a factor of 10 to 10 -5 、10 -6 、10 -7 M.
[0097] (4) Ellman's reagent
[0098] W...
Embodiment 2
[0107] Example 2——The activity of recombinant AChE protein expressed by yeast is inhibited by trace amounts of chlorpyrifos
[0108] 1. Solution
[0109] (1) Chlorpyrifos solution
[0110] Weigh 0.175g of chlorpyrifos (MW=350.6) crystals and dissolve in 5ml of acetone. At this time, the concentration of chlorpyrifos is 0.1M. Dilute the solution stepwise by a factor of 10 to 10 -5 、10 -6 、10 -7 M.
[0111] All the other solutions and reagent formulations are the same as in Example 1.
[0112] 2. Operation steps
[0113]Operation is basically the same as in Example 1. Dissolve chlorpyrifos crystals with acetone to prepare a concentration of 10 -5 , 10 -6 , 10 -7 M's solution. Take 3 μl and 50 μl of crude enzyme solution, mix them, and incubate (25°C, 15min). At the same time, a control group was set up, which was formed by mixing 3 μl of acetone and 50 μl of crude enzyme solution. Add the mixed solution into the microplate well containing 250 μl Ellman reagent, shak...
Embodiment 3
[0116] Example 3——The activity of the recombinant AChE protein expressed by yeast is inhibited by a small amount of pirimicarb
[0117] 1. Solution
[0118] (1) Anti-pirimicarb solution
[0119] Weigh 0.119g of pirimicarb (MW=238.33) crystals and dissolve it in 5ml of acetone. At this time, the concentration of pirimicarb is 0.1M. Dilute the solution stepwise by a factor of 10 to 10 -5 、10 -6 、10 -7 M.
[0120] All the other solutions and reagent formulations are the same as in Example 1.
[0121] 2. Operation steps
[0122] Operation is basically the same as in Example 1. Dissolve anti-pirimicarb crystals with acetone, and prepare a concentration of 10 -5 , 10 -6 , 10 -7 M's solution. Take 3 μl and 50 μl of crude enzyme solution, mix them, and incubate (25°C, 15min). At the same time, a control group was set up, which was formed by mixing 3 μl of acetone and 50 μl of crude enzyme solution. Add the mixed solution into the microwell containing 250 μl Ellman reagent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com