Prepn process of peptide HIV proteinase inhibitor
A technology of protease inhibitors and peptides, which is applied in the field of preparation of peptide HIV protease inhibitors, can solve the problems of low yield, many steps, and difficult sources of raw materials, and achieve high yield, mild process conditions, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] step 1):
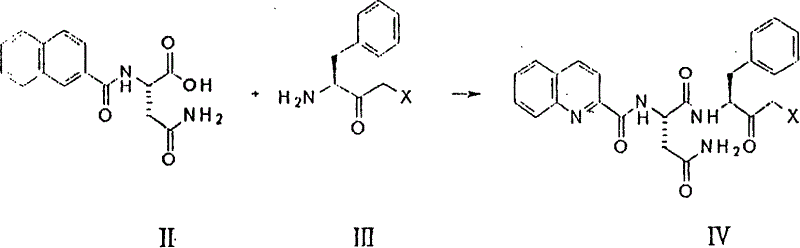
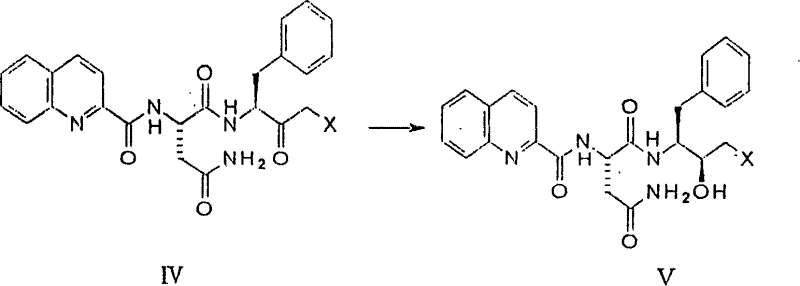
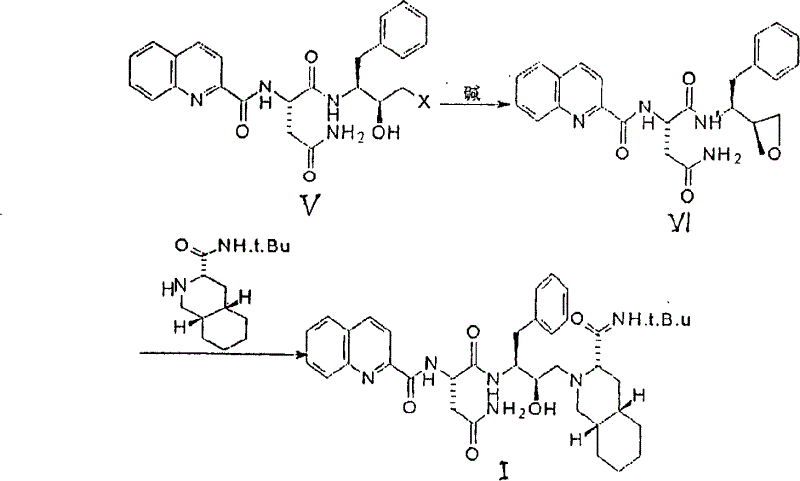
[0042] Dissolve 199mg of 3-amino-4-phenyl-4-phenyl-1-chloro-2-butanone and 287mg of N-(quinoline-2-formyl)-L-asparagine in 15ml of tetrahydrofuran solution, cool to -10°C, then add 225mg of dicyclohexylcarbodiimide, 164mg of 3-hydroxybenzotriazole and 126mg of N-ethylmorpholine, stir the reaction at -10°C for 2hr, stir at room temperature for 20hr, then add acetic acid Dilute with ethyl ester and filter. The filtrate was washed with saturated sodium bicarbonate solution and saturated saline solution, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The residue was separated by chromatography (mobile phase: methanol:dichloromethane=1:9) to obtain 261 mg of 3(S) -[[N-(quinoline-2-formyl)-L-asparaginyl]amino]-4-phenyl-1-chloro-2-butanone.
[0043] N-(quinoline-2-formyl)-L-asparagine used as starting material was prepared as follows:
[0044] Dissolve 172mg of L-asparagine and 200mg of triethylamine in 15mL of e...
Embodiment 2
[0050] The same method as in Example 1 was adopted, wherein the solvent in step (1) was replaced by dimethylformamide for tetrahydrofuran, to obtain 310 mg of the target product.
Embodiment 3
[0052] Adopt the method identical with embodiment 1, wherein: the borohydride of the alkali metal of step (2) is NaBH 4 Obtained 308 mg of the target product. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com