Chitose sulfuric ester metal complex and its preparing method
A technology of chitosan sulfate and metal complexes, which is applied in the field of marine chemical engineering, can solve the problems of severe degradation of polysaccharide molecules, low degree of sulfation, and impact on product yield, and achieves improved esterification efficiency and good water solubility. performance and the effect of expanding the application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
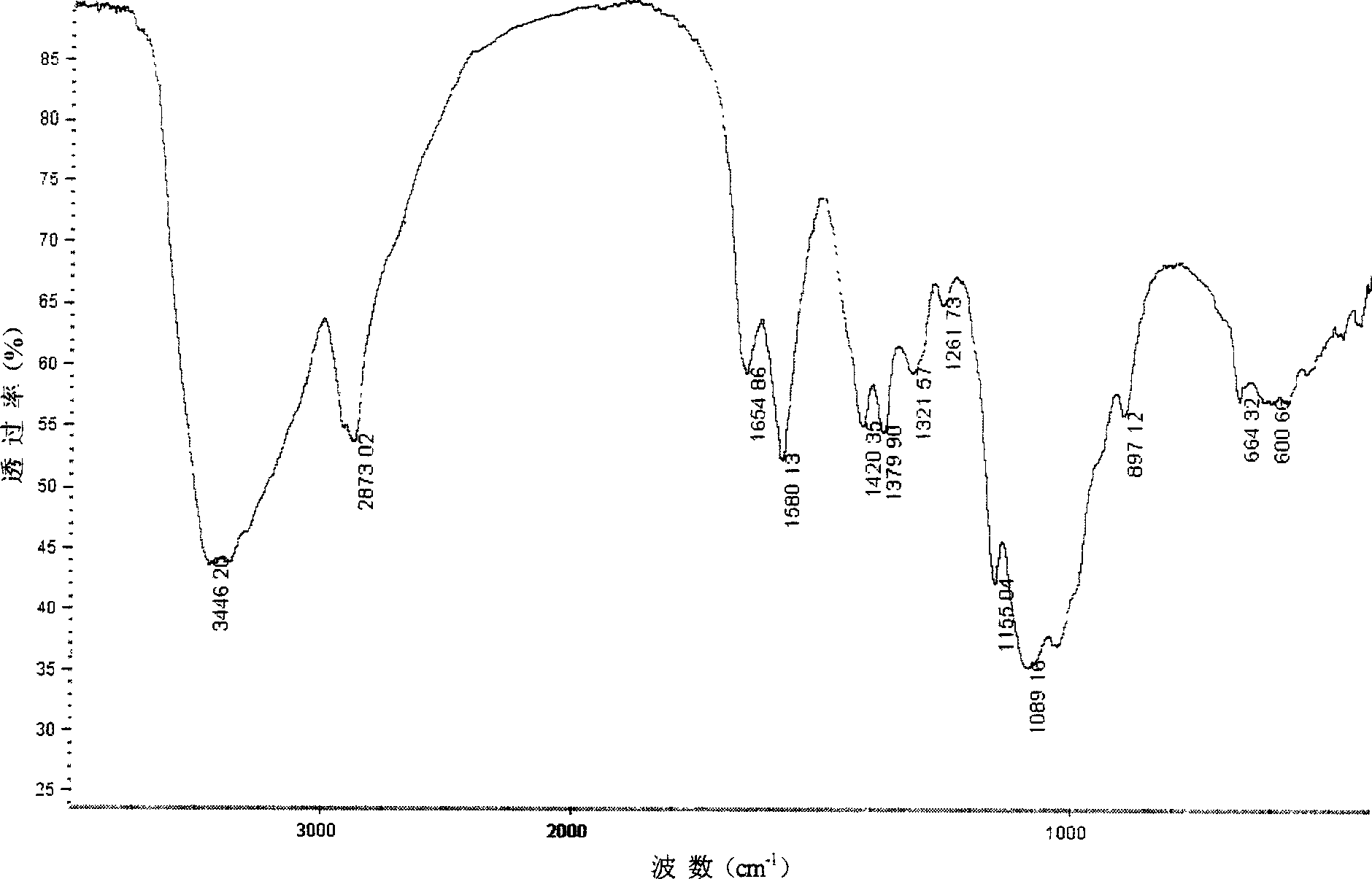
[0037] 4g chitosan (Mw=7.6×10 5 , the degree of deacetylation is 84.1%) after dissolving with 1% acetic acid, adding an equimolar amount of CuSO 4 Aqueous solution, stirred for 2 hours, adjusted the pH of the solution to 4-6 with dilute ammonia water, continued to stir for 4 hours, precipitated with 3-4 times the volume of acetone, filtered, washed, dried, and pulverized to obtain chitosan copper (II) complex , Take 2g of the above complex, suspend it in 50mL formamide, add 30mL DMF-SO 3 , cover the lid, place the reactor in a microwave oven, react at 850w microwave power for 2min (intermittent operation), add 3-5 times the amount of 95% ethanol to form a precipitate, place it in a refrigerator at 4°C for 1h, filter it with suction, add 20mL distilled water to dissolve , adjust the pH to neutral with 1mol / L NaOH solution, put it into a dialysis bag, dialyze with distilled water for 3-4 days, concentrate the dialysate to 10-20mL by rotary evaporation, and obtain chitosan sulfa...
Embodiment 2
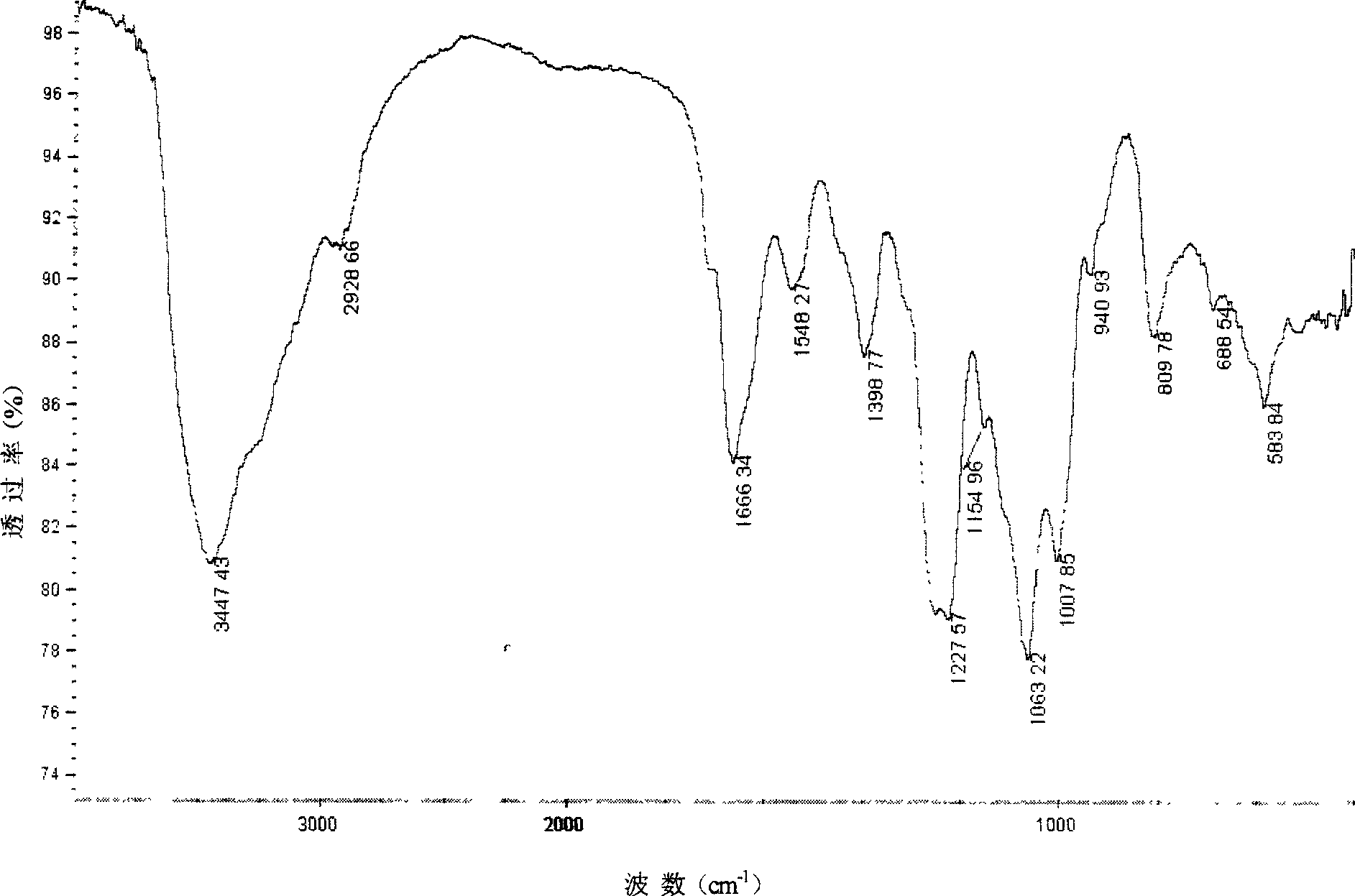
[0041] 4g chitosan (Mw=7.6×10 5 , the degree of deacetylation is 84.1%) after dissolving with 1% formic acid, adding an equimolar amount of ZnSO 4 Aqueous solution, stirred for 2 hours, adjusted the pH of the solution to 4-6 with dilute ammonia water, continued to stir for 4 hours, precipitated with 3-4 times the volume of acetone, filtered, washed, dried, and crushed to obtain chitosan zinc (II) complex , Take 2g of the above complex, suspend it in 100mL dimethylformamide, add 60mL DMF-SO 3 , cover the lid, place the reactor in a microwave oven, react for 3 minutes under 680W microwave power (intermittent operation), add 3-5 times the amount of 95% ethanol to form a precipitate, place it in a refrigerator at 4°C for 1 hour, filter with suction, add 20mL of distilled water solution, adjust the pH to neutral with 1mol / L NaOH solution, put it in a dialysis bag, dialyze with distilled water for 3-4 days, concentrate the dialysate to 10-20mL by rotary evaporation, and obtain chit...
Embodiment 3
[0044] Embodiment 3 (low molecular weight chitosan complex)
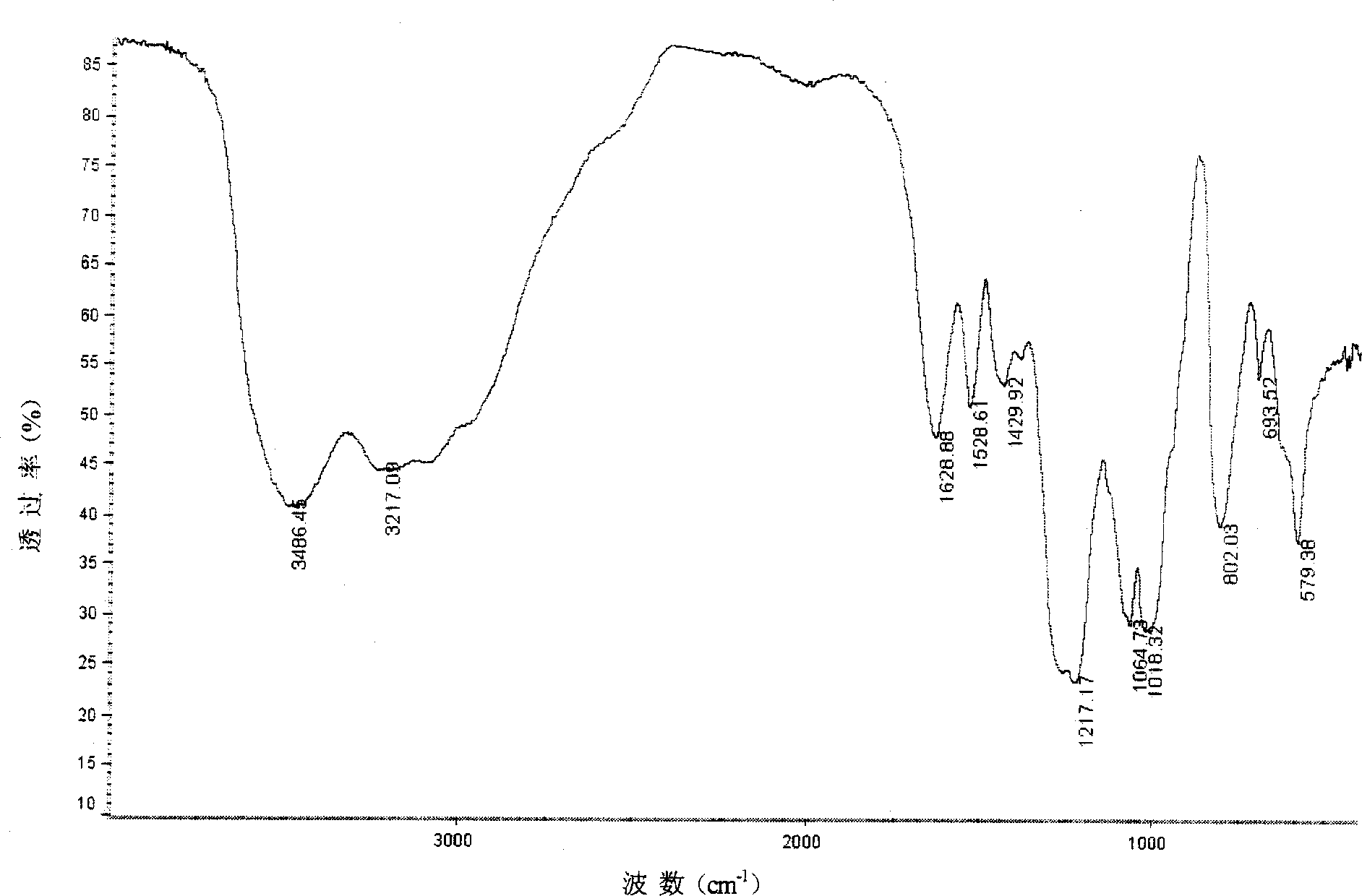
[0045] 4g low molecular weight chitosan (Mw=2.0×10 4 , the degree of deacetylation is 85.4%) was dissolved in 1% acetic acid, and an equimolar amount of CuSO was added 4 Aqueous solution, stirred for 2 hours, adjusted the pH of the solution to 4-6 with dilute ammonia water, continued to stir for 6 hours, precipitated with 3-4 times the volume of acetone, filtered, washed, dried, and pulverized to obtain the chitosan copper (II) complex , Take 2g of the above complex, suspend it in 60mL formamide, add 20mL DMF-SO 3 , cover the lid, place the reactor in a microwave oven, react at 510W microwave power for 4min (intermittent operation), add 3-5 times the amount of ethanol to form a precipitate, place it in a refrigerator at 4°C for 3h, filter it with suction, add 20mL distilled water to dissolve, and use 1mol / L NaOH solution to adjust the pH to neutral, put it into a dialysis bag, dialyze with distilled water for 3-4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com