Preventive or remedy for diseases caused by hyperglycemia
A technology for hyperglycemia and postprandial hyperglycemia, applied to metabolic diseases, medical preparations containing active ingredients, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] 5-Hydroxy-3-methyl-2-(4-[3-(3-pyridylmethyl)ureido]benzyl}-phenyl β-D-galactopyranoside
[0115] To the 2-(4-aminobenzyl)-5-methoxycarbonyloxy-3-methylphenyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyran To a solution of glycoside (0.25g) and pyridine (0.043ml) in dichloromethane (10ml) was added 4-nitrophenylchloroformate (90mg), and the mixture was stirred at room temperature for 12 hours. Then, 3-aminomethylpyridine (0.045ml) and triethylamine (0.11ml) were added to the reaction mixture, and the mixture was stirred at room temperature for 5 hours. The reaction mixture was concentrated under reduced pressure, and the residue was dissolved in methanol (8 ml). To this solution was added sodium methoxide (28% in methanol, 0.39 ml), and the mixture was stirred at room temperature for 2 hours. The reaction mixture was concentrated under reduced pressure, and the residue was washed successively in ODS (washing solvent: distilled water, eluent: methanol) and VARIAN BOND ELUT ...
Embodiment 2
[0149] 3-(β-D-glucopyranoseoxy)-4-{[4-(2-guanidinoethoxy)-2-methylphenyl]methyl}-5-isopropyl-1H-pyridine azole
[0150] To 3-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranoseoxy)-4-{[4-(2-aminoethoxy)-2-methylbenzene Base] methyl}-5-isopropyl-1H-pyrazole (0.6 grams) in tetrahydrofuran (5ml)-N, N-dimethylformamide (1ml) solution, add N-(benzyloxycarbonyl) - 1H-pyrazole-1-carboxamidine (N-(benzyloxycarbonyl)-1H-pyrazole-1-carboxamidine) (1.89 g), and the mixture was stirred at 60°C for 20 hours. The reaction mixture was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (eluent: n-hexane / ethyl acetate=1 / 1-ethyl acetate-ethyl acetate / ethanol=10 / 1) to obtain 3-(2 , 3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)-4-({4-[2-(N′-benzyloxycarbonyl-guanidino)ethoxy] -2-Methylphenyl}methyl)-5-isopropyl-1H-pyrazole (0.31 g). This material was dissolved in methanol (6ml). To the methanol solution was added sodium methoxide (28% methanol solution,...
Embodiment 3
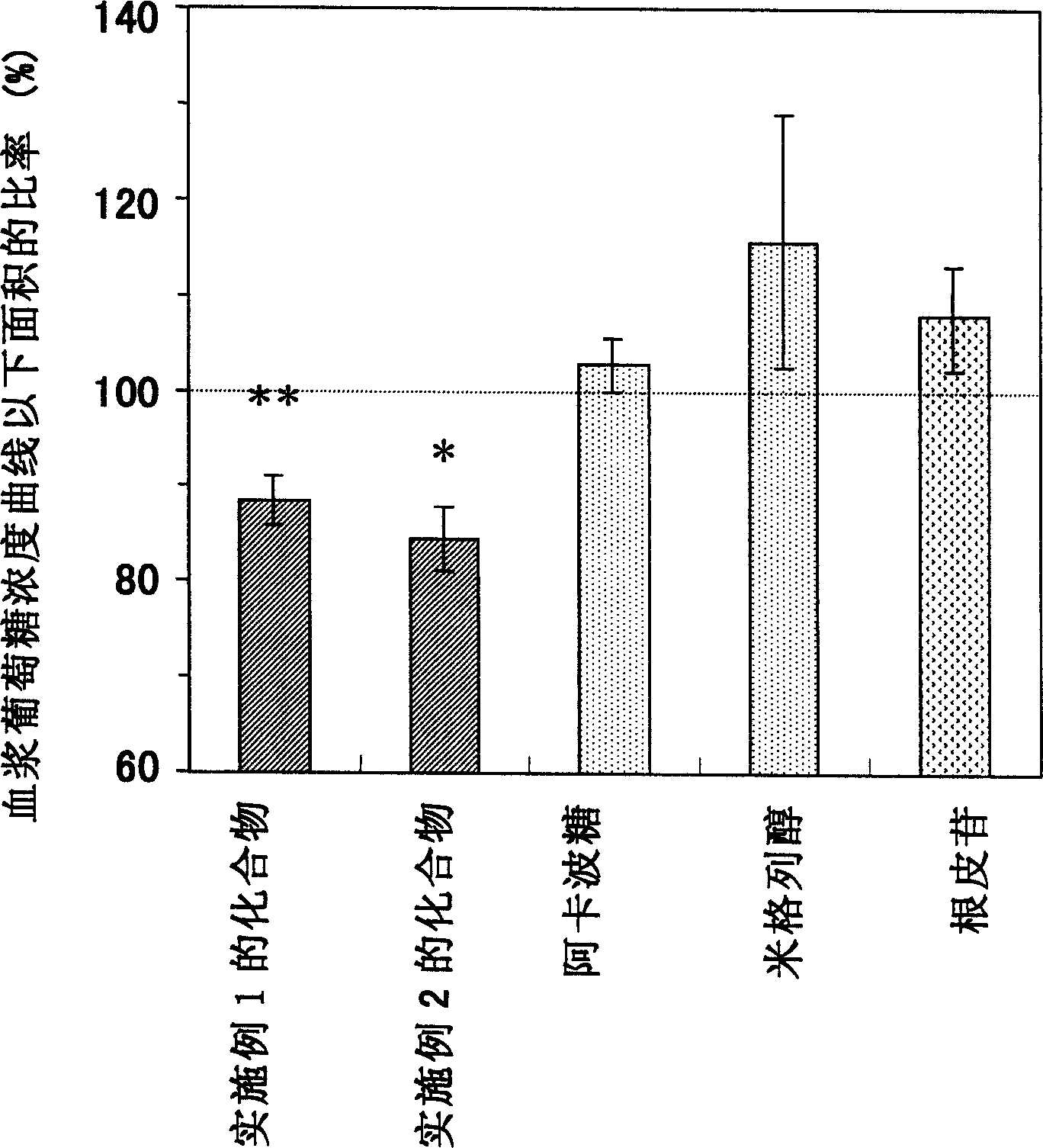
[0154] Testing for inhibition of SGLT1 activity in rats
[0155] 1) Cloning and construction of vector expressing rat SGLT1
[0156] Rat kidney cDNA library (QUICK-Cline TM cDNA; Clontech) was used as a template to amplify a DNA fragment of 111-2203bp encoding rat SGLT1 (accession number: M16101, reported by Kasahara et al.) by PCR method, and inserted into the SrfI site of pCMV-Script (Stratagene). The inserted DNA sequence exactly matches the previously reported sequence at the amino acid level.
[0157] 2) Establish a cell line stably expressing rat SGLT1
[0158] The expression vector of rat SGLT1 was digested with MluI to form linear DNA. This linear DNA was transferred into CHO-K1 cells by lipofection (Superfect transfection reagent: QIAGEN). Neomycin-resistant cell lines were selected by culturing in a medium containing G418 (1 mg / mL, LIFE TECHNOLOGIES), and then the activity of antagonizing methyl-α-D-glucopyranose uptake was measured by the following method. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com