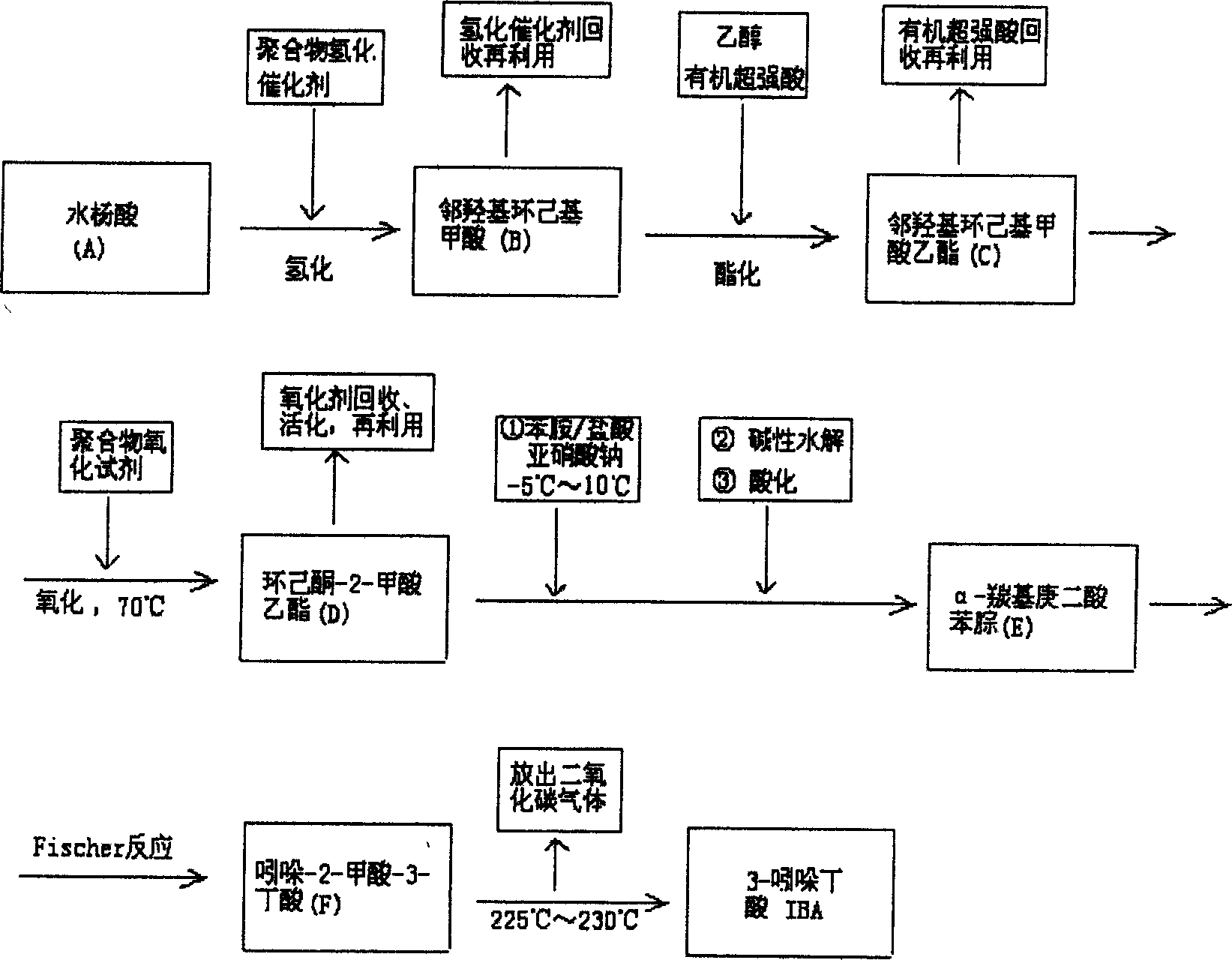
Synthesis of plant growth hormone 3-indolebutyric acid
A technology of plant auxin and indole butyric acid, applied in the direction of organic chemistry, can solve the problems of harsh process conditions, large environmental pollution, high cost of raw materials, etc., and achieve the effect of simple synthesis process, avoiding environmental pollution, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Example 1. Under normal temperature and pressure, charge about 10 liters of hydrogen to the hydrogen metering bottle of the hydrogenation unit (until it is full), add 300ml (5.2mol) absolute ethanol, 13.8g (0.1mol) salicylic acid in the there-necked bottle and 3g of silicon dioxide-poly-γ-diphenylphosphinopropylsiloxane-platinum complex (Si-P-Pt), open the valve between the hydrogen metering device and the there-necked bottle, and make the hydrogen Press down to contact with the reaction solution, and after 30 minutes, 6.7 liters of hydrogen gas was absorbed. Separate the hydrogenation catalyst, then pour the reaction solution into a three-necked bottle, add 5g of 10-15 mesh perfluorosulfonic acid resin at the same time, install a reflux condenser and a thermometer, and reflux for 1.5 hours, then separate the perfluorosulfonic acid resin and recover it. Ethanol was distilled off again to obtain the product ethyl o-hydroxycyclohexyl carboxylate with a yield of 98.7%.
...
example 2
[0019] Example 2. hydrogenation unit, charging capacity and operating steps are the same as example 1, and the polymer hydrogenation catalyst is changed into 3g carbon fiber diphenylphosphine-palladium complex, after hydrogenation, carry out catalytic esterification again, obtain o-hydroxycyclohexyl formate ethyl ester, The yield was 95.5% based on salicylic acid.
example 3
[0020] Example 3. The polymer hydrogenation catalyst in Example 1 was replaced with 3g polystyrene-diphenylphosphine-palladium complex, reacted as above, and the calculated yield was 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com