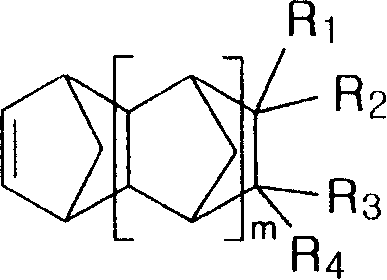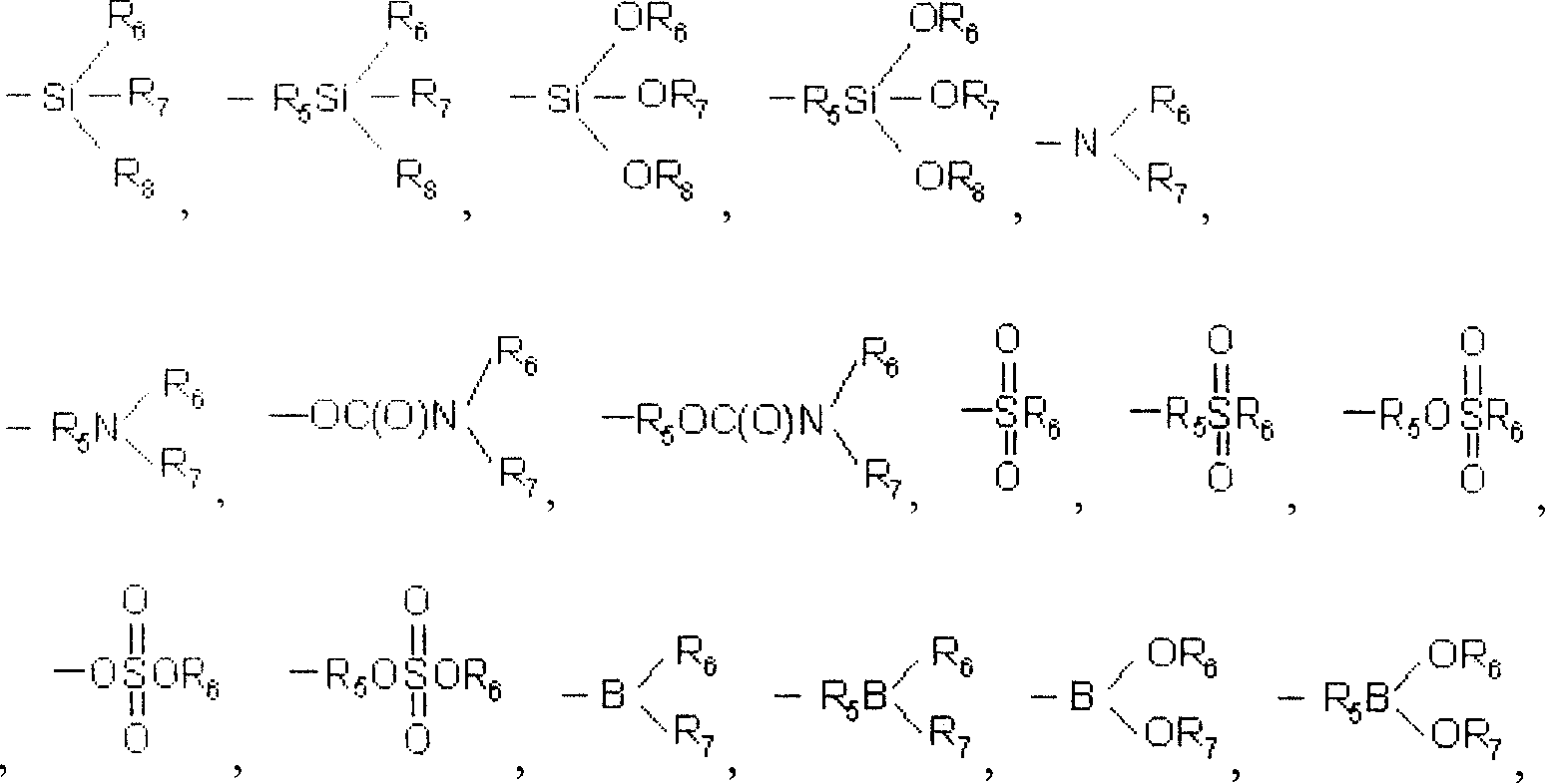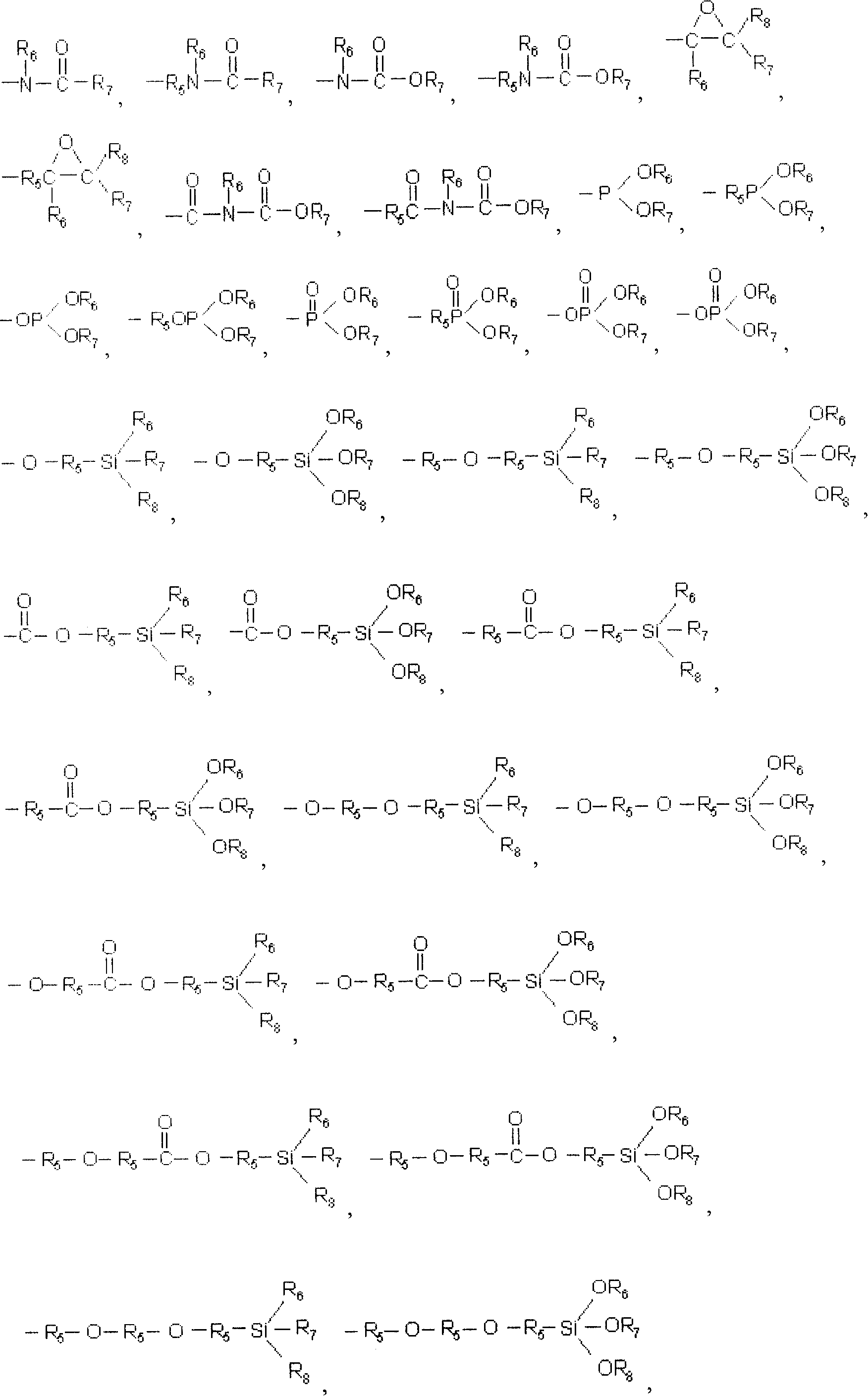Negative C-plate type optical anisotropic film comprising poly cycloolefin and method for preparing the same
一种光学各向异性、聚环烯烃的技术,应用在光学、光学元件、非线性光学等方向,能够解决催化剂中毒等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention provides a negative C-plate type optically anisotropic film, especially a film comprising a cycloolefin addition polymer prepared by addition polymerization of a norbornene-based monomer, a method for preparing the same, and a film comprising the film LCD Monitor.
[0056] The cycloolefin addition polymer obtained by addition polymerization of a norbornene-based monomer of the present invention includes a homopolymer obtained by addition polymerization of a norbornene-based monomer represented by Chemical Formula 1 below, and Another copolymer obtained by addition copolymerization of monomers:
[0057] chemical formula 1
[0058]
[0059] In Chemical Formula 1,
[0060] m represents an integer from 0 to 4;
[0061] R 1 , R 2 , R 3 and R 4 Hydrogen alone or together; Halogen; C 1 to C 20 Alkyl, alkenyl or vinyl of linear or branched chain structure; C 4 to C 12 Hydrocarbyl substituted or unsubstituted cycloalkyl; C 6 to C 40 Hydrocar...
preparation Embodiment 1
[0158] Preparation Example 1: Polymerization of norbornene carboxylate methyl ester
[0159] The norbornene carboxylate methyl ester monomer and purified toluene were added to the polymerization reactor in a weight ratio of 1:1.
[0160] 0.01 mol% (relative to monomer content) Pd(acac) dissolved in toluene 2 and 0.01 mol% (relative to the monomer content) of tricyclohexylphosphine as a catalyst, and 0.02 mol% (relative to the monomer content) dissolved in CH 2 Cl 2 Tetrakis(pentafluorophenyl) borate dimethylaniline in is added to the reactor as a cocatalyst. The reaction was carried out under stirring at 80°C for 20 hours.
[0161] After the reaction is finished, the reaction mixture is added to excess ethanol to obtain a white copolymer precipitate. The precipitate was filtered with a glass funnel, and then the collected copolymer was dried in a vacuum oven at 65° C. for 24 hours to obtain a norbornene carboxylate methyl ester polymer (PMeNB).
preparation Embodiment 2
[0162] Preparation Example 2: Polymerization of Norbornene Carboxylate Butyl Ester
[0163] The butyl norbornene carboxylate monomer and purified toluene were added to the polymerization reactor at a weight ratio of 1:1.
[0164] 0.01 mol% (relative to monomer content) Pd(acac) dissolved in toluene 2 , and 0.01 mol% (relative to monomer content) of tricyclohexylphosphine as a catalyst, 0.02 mol% (relative to monomer content) dissolved in CH 2 Cl 2 Tetrakis(pentafluorophenyl) borate dimethylaniline in is added to the reactor as a cocatalyst. The reaction was carried out under stirring at 80°C for 20 hours.
[0165] After the reaction was completed, the reaction mixture was added to excess ethanol to obtain a white copolymer precipitate. The precipitate was filtered with a glass funnel, and then the collected copolymer was dried in a vacuum oven at 65° C. for 24 hours to obtain a norbornene carboxylate butyl ester polymer (PBeNB).
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| visible light transmittance | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com