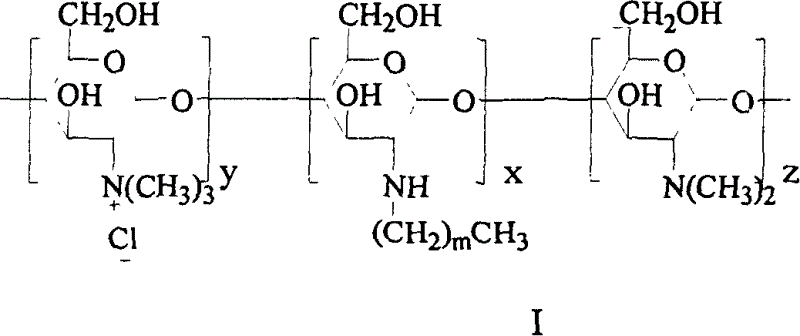
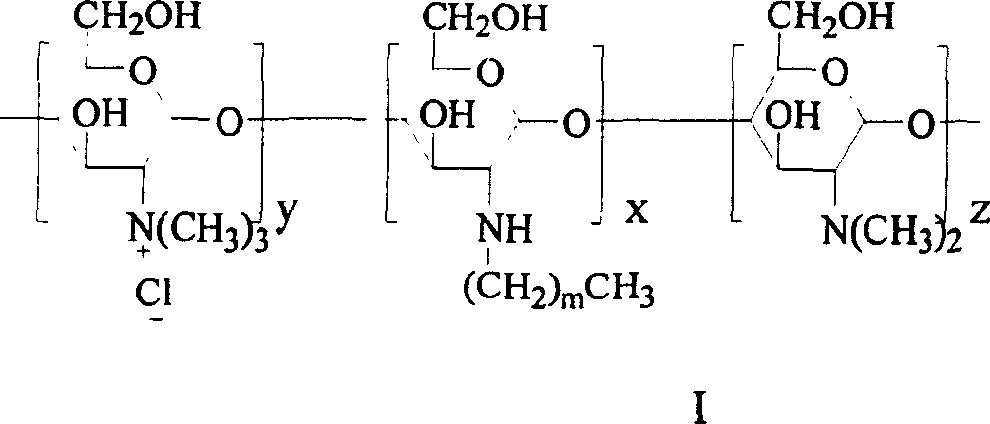
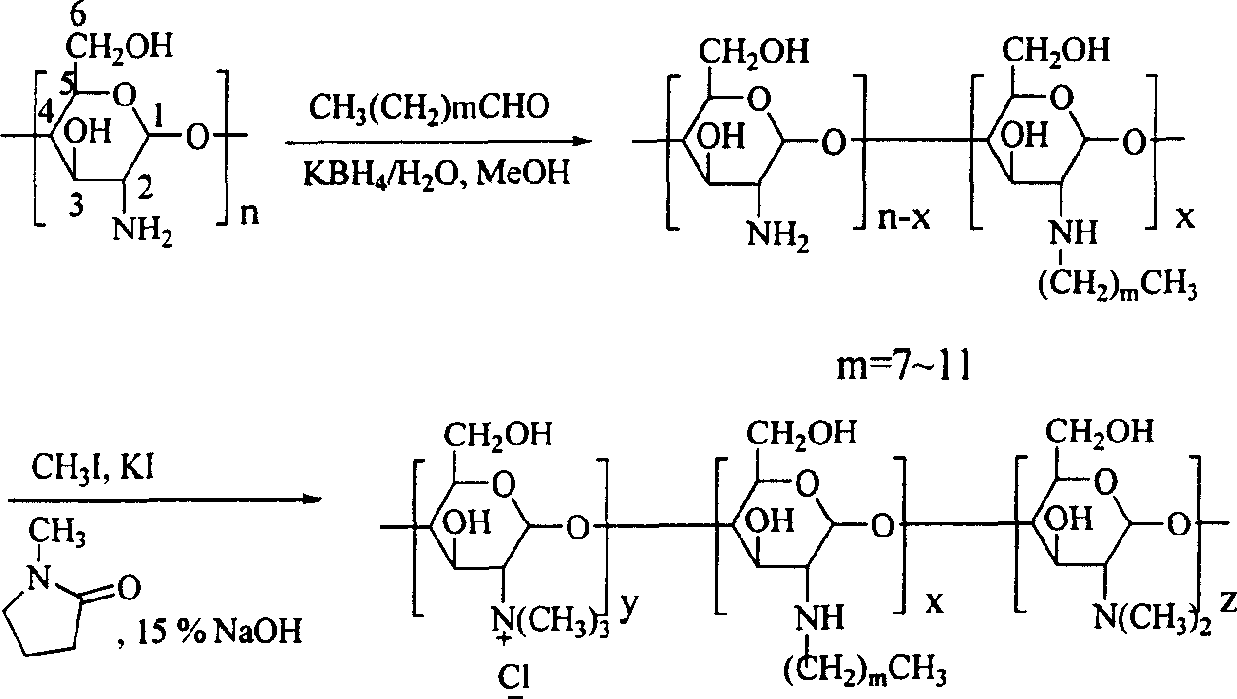
Quaterisation chitosan derivatives, preparation method and medicinal preparation containing the derivatives
A technology of quaternized chitosan and derivatives, applied in the field of polymer chemistry, can solve the problem of severe allergic reactions in patients, achieve good solubilization effect, improve treatment effect, and reduce phagocytosis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Preparation of N-octyl chitosan (OCS)
[0034] Add 12g chitosan and 34.0ml n-octylaldehyde into 250ml methanol, stir at 30°C for 5h, then add KBH in batches 4(a total of 6g / 60ml), after stirring overnight, filtered, washed the filter cake repeatedly with water and hot methanol, and vacuum-dried at 50°C to obtain 11.3g of light yellow powder OCS (octyl substitution degree was 10%).
[0035] 2. Preparation of N-alkyl-N-quaternized chitosan (OTMCS1)
[0036] 0.96g N-octyl chitosan was placed in a 100ml three-neck flask, and 15ml N-methylpyrrolidone, 2.4g KI, 5ml 15% NaOH aqueous solution and 5.2ml CH 3 I, under full stirring, heat up to 60°C for 1h, cool to room temperature, centrifuge for 30min (1000rpm), collect the lower layer solid (I) and dissolve it in distilled water, dialyze for 5d, filter the insoluble matter and freeze-dry the filtrate to obtain light yellow N - 0.34 g of octyl-N-quaternized chitosan (OTMCS1) (substitution degree of quaternary amine is 53%)....
Embodiment 2
[0050] 1. Preparation of N-decyl chitosan (DCS)
[0051] With decanal and chitosan reaction, preparation method is the same as the preparation method of N-octyl chitosan among the embodiment 1.
[0052] 2. Preparation of N-decyl-N-quaternized chitosan (DTMCS1,)
[0053] It is prepared by reacting N-decyl chitosan and methyl iodide, and the preparation method is the same as that of OTMCS1 in Example 1.
[0054] FT-IR: 2953, 2872, 1080, 1035, 810cm -1
[0055] 1 H NMR (500MHz, D 2 O): 5.4 (H 1 ), 4.3~3.4(H 3 , H 4 , H 5 , H 6 ), 3.0~3.3(N(CH 3 ) 3 ,-NH-CH 2 -(CH 2 ) 8 -CH 3 , H 2 ), 2.6(N(CH 3 ) 2 , 1.3~2.3(-NH-CH 2 -(CH 2 ) 8 -CH 3 ), 0.82 (-NH-CH 2 -(CH 2 ) 8 -CH 3 ).
[0056] 13 C NMR (500MHz, D 2 O): 99.6 (C 1 ), 77.5 (C 4 ), 77(C 5 ), 68.4 (C 3 ), 61.1 (C 6 ), 58.6 (C 2 ), 52.9(N(CH 3 ) 3 ), 45.0 (-NH-CH 2 -(CH 2 ) 8 -CH 3 ), 40.9 (N (CH 3 ) 2 ), 34.3 (-NH-CH 2 -(CH 2 )8 -CH 3 ), 20.1~23.9 (-NH-CH 2 -(CH 2 ) 8 -CH 3 ).
[0...
Embodiment 3
[0065] 1. Preparation of N-dodecyl-chitosan (LCS)
[0066] With lauric aldehyde and chitosan reaction, preparation method is the same as the N-octyl chitosan of embodiment 1.
[0067] 2. Preparation of N-decyl-N-quaternized chitosan (LTMCS1)
[0068] It is prepared by reacting N-dodecyl chitosan and methyl iodide, and the method is the same as that of OTMCS1 in Example 1.
[0069] FT-IR: 2950, 2850, 2950, 2860, 1462, 1385, 800em -1 .
[0070] 1 H NMR (500MHz, D 2 O): 5.42 (H 1 ), 4.3~3.4(H 3 , H 4 , H 5 , H 6 ), 3.0~3.3 (-NH-CH 2 -(CH 2 ) 8 -CH 3 , N(CH 3 ) 3 , H 2 ), 2.5(N(CH 3 ) 2 ), 1.0~2.5(-NH-CH 2 -(CH 2 ) 8 -CH 3 NOCOCH 3 ), 0.8(-NH-CH 2 -(CH 2 ) 8 -CH 3 ).
[0071] 13 C NMR (500MHz, D 2 O): 97.5 (C 1 ), 77.1 (C 4 ), 75.7 (C 5 ), 65.9 (C 3 ), 62.1 (C 6 ), 55.3 (C 2 ), 53.7(N(CH 3 ) 3 ), 48.0 (-NH-CH 2 -(CH 2 ) 8 -CH 3 ), 41.1 (N (CH 3 ) 2 ), 22.3~34.2; (-NH-CH 2 -(CH 2 ) 8 -CH 3 ), 13.5~13.7(-NH-CH 2 -(CH 2 ) 8 -CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com