Novel fusion protein production and uses
A technology of fusion protein and thioredoxin, which is applied in the field of human acidic fibroblast growth factor variant fusion protein and its preparation, biotechnology and pharmaceuticals, and can solve the problems of poor stability of aFGF prototype
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
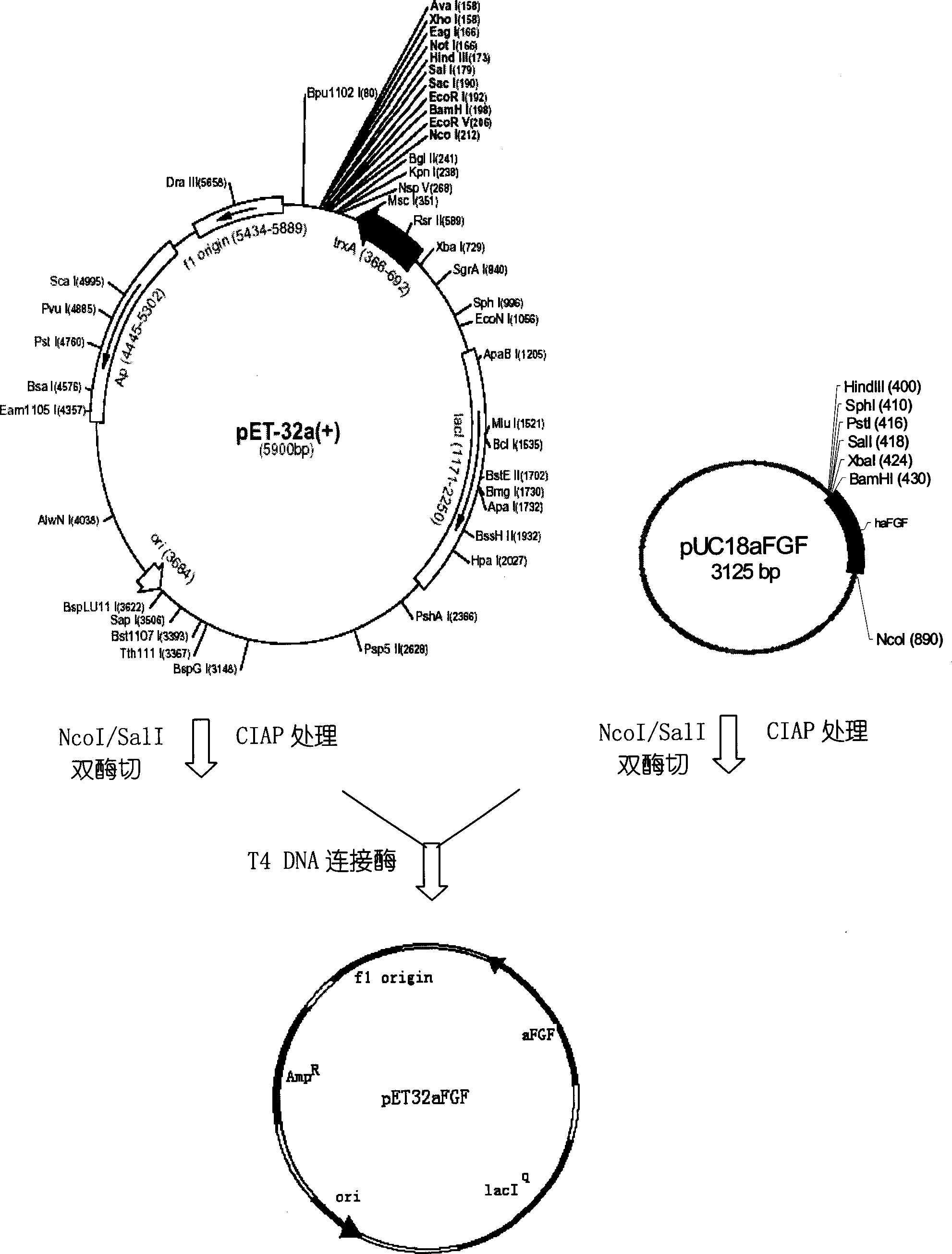
[0027] Construction of Genetic Engineering Bacteria Recombinantly Expressing Human Acidic Fibroblast Growth Factor Variant Fusion Protein
[0028] (1) Artificially synthesize a double-stranded DNA gene encoding human aFGF 1-140 amino acid residues, and add an NcoI site at the 5' end, introduce an Ala residue at the N-terminus of the prototype haFGF, and add a SalI site at the 3' end, The sequence is as follows:
[0029] 5’…catggctttcaacctgccgccgggtaactacaaaaaaccgaaactgctgtactcctccaacggtggtcac
[0030] ttcctgcgtatcctgccggacggtaccgttgacggtacccgtgaccgttccgaccagcacatccagctgcagctg
[0031] tccgctgaatccgttggtgaagtttacatcaaatccaccgaaaccggtcagtacctggctatggacaccgacggt
[0032] ctgctgtacggttcccagaccccgaacgaagaatccctgttcctggaacgtctggaagaaaacccatacaacacc
[0033] tacatctccaaaaaacacgctgaaaaaaactggttcgttggtctgaaaaaaaacggttcctccaaacgtggtccg
[0034] cgtacccactacggtcagaaagctatcctgttcctgccgctgccggtttcctccgactaagtcgac...3'
[0035] The resulting DNA fragment was cloned with NcoI / SalI site ...
Embodiment 2
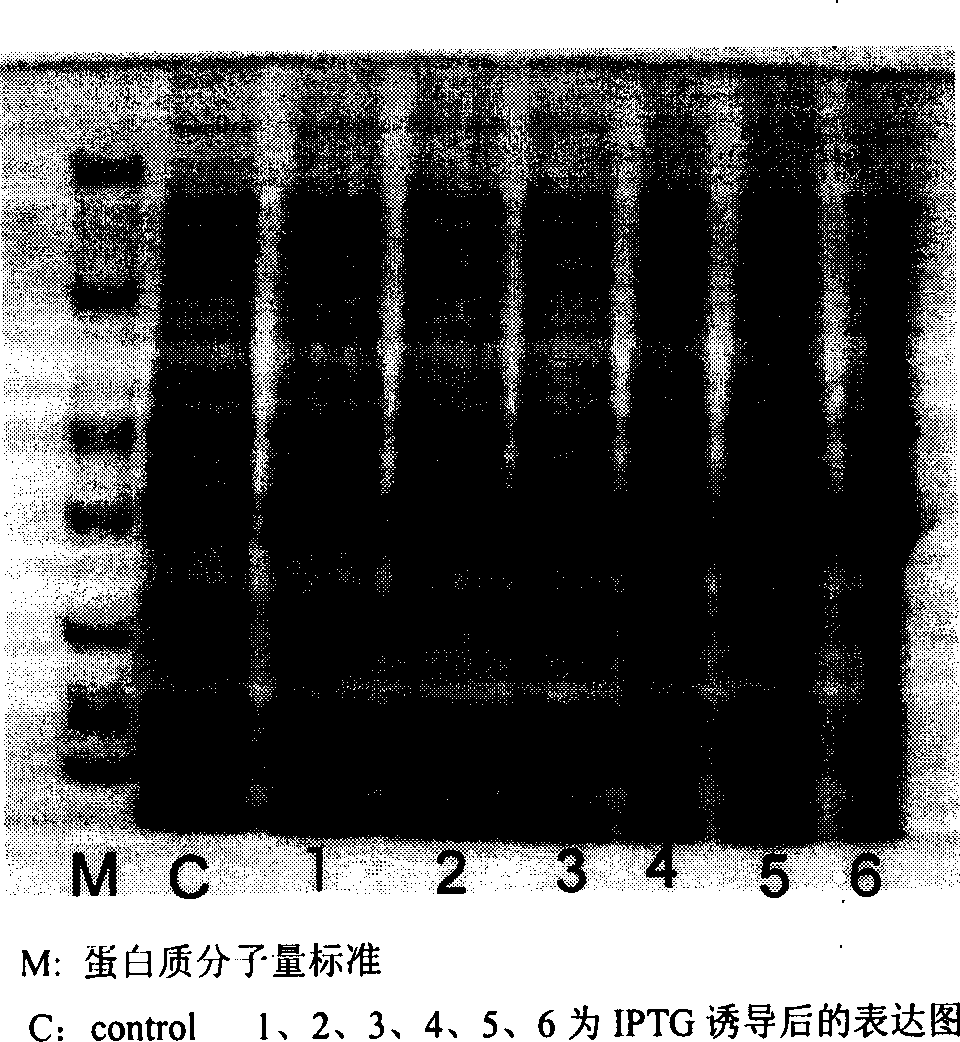
[0042] Purification of recombinant human acidic fibroblast growth factor variant fusion protein
[0043] The fermented liquid obtained from the fermentation culture was centrifuged in a refrigerated large-capacity centrifuge (7000rpm, 5min), and the bacteria were collected and washed three times with buffer solution (25mmol / L Tris, 0.15mol / L NaCl, pH8.0), and the culture medium was removed. the remains.
[0044] The fermented cells collected by centrifugation were uniformly suspended with buffer solution (25mmol / L Tris, 0.15mol / LNaCl, 1mmol / L EDTA, pH8.0) at a ratio of 20% (w / v), and cooled in an ice bath. Adjust the working pressure of APV 1000 homogenizer to destroy bacteria.
[0045] After the bacterial cell was crushed by high-pressure homogenization, the crude inclusion body was obtained by high-speed centrifugation (9000 rpm, 30 min). "7 mol / L guanidine hydrochloride, 50 mmol / L Tris, pH8.0" was used as the dissolution buffer of rhaFGF inclusion bodies. The rhaFGF incl...
Embodiment 3
[0054] Liquid and lyophilized preparations of Tioredoxin-aFGF fusion protein
[0055] The prescription of external recombinant human acidic fibroblast growth factor (fusion protein) lyophilized preparation is:
[0056] Fusion protein 1×10 5 IU (or 0.2mg)
[0057] Tris 3.02mg
[0058] NaCl 9mg
[0059] Human Albumin 10mg
[0060] Packed in 1.0ml / cartridge and then freeze-dried, that is, each finished product contains 1×10 5 IU (or 0.2 mg) rhaFGF, 9 mg NaCl, 3.02 mg Tris and 10 mg human albumin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com