Process for preparing N-amino piperidine hydrochloride
A technology for the preparation of aminopiperidine, applied in the field of preparation of N-aminopiperidine hydrochloride, capable of solving problems such as long reaction time, high temperature and pressure, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
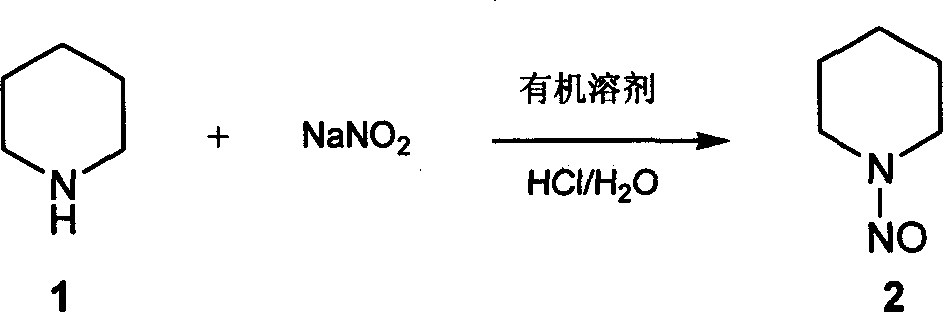
[0014] Dissolve 39.6ml of piperidine in 40ml of water, under ice bath cooling, slowly add 85ml of concentrated hydrochloric acid dropwise, then add 250ml of organic solvent ethyl acetate, dissolve 27.6g of sodium nitrite in water, drop into the reaction solution, add dropwise The time was 2 hours, and the ice bath continued to stir for 30 minutes, and then 13.8g of sodium nitrite was dissolved in water and dropped into the reaction solution; naturally rose to room temperature and stirred, and after 2 hours of reaction, the reaction color changed from green to yellow, and the reaction was stopped. The layers were separated, the aqueous layer was back-extracted twice with ethyl acetate, the organic layers were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 38.7 g of the compound nitrosopiperidine as a yellow oily liquid with a yield of 84.8 %;
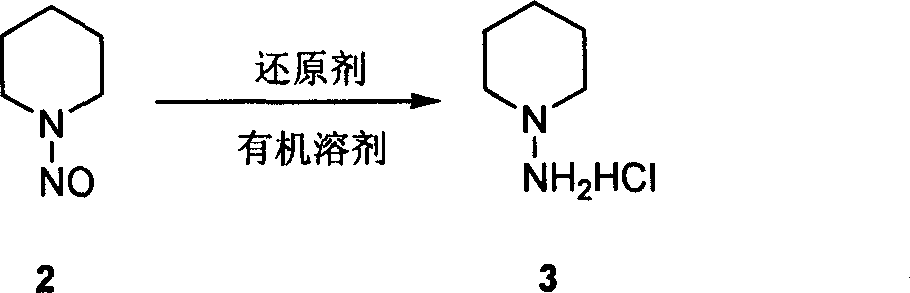
[0015] In a dry 250ml single-necked bottle, add 50ml of anhydro...
Embodiment 2
[0017] Dissolve 120ml of piperidine in 120ml of water, under ice bath cooling, slowly add 260ml of concentrated hydrochloric acid dropwise, then add 750ml of organic solvent chloroform, dissolve 82.8g of sodium nitrite in water, drop into the reaction solution, dropwise for 1 hour 40 Continue to stir in the ice bath for 30 minutes, then dissolve 41.6g of sodium nitrite in water, drop into the reaction solution; naturally rise to room temperature and stir, after 2.5 hours of reaction, the reaction color turns from green to yellow, stop the reaction, and separate layers , the aqueous layer was back-extracted twice with chloroform, the combined organic layers were washed once with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 115.8 g of compound nitrosopiperidine as a yellow oily liquid with a yield of 83.49%;
[0018] In a dry 1000ml single-necked bottle, add 150ml of ether and 3.42g of lithium aluminum tetrahydride under ice bath, di...
Embodiment 3
[0020] Dissolve 39.6ml of piperidine in 40ml of water, under ice bath cooling, slowly add 85ml of concentrated hydrochloric acid dropwise, then add 250ml of organic solvent toluene, dissolve 27.6g of sodium nitrite in water, drop into the reaction solution, dropwise for 2 After 3 hours, the ice bath continued to stir for 30 minutes, then 13.8g of sodium nitrite was dissolved in water and dropped into the reaction solution; naturally rose to room temperature and stirred, and after 3 hours of reaction, the reaction color changed from green to yellow, the reaction was stopped, and the layers were separated. , the aqueous layer was back-extracted twice with toluene, the organic layers were combined, and washed once with saturated brine; dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 38.7 g of compound nitrosopiperidine as a yellow oily liquid, with a yield of 84.8%;
[0021] Add 1.68g of iron filings, 0.5ml of concentrated hydrochloric acid and 20ml of w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com