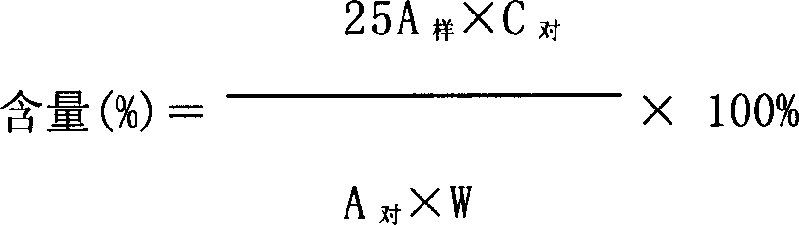Quality control method of cbinese medicinal preparation
A quality control method and preparation technology, which is applied in the quality control of Gongyanping capsules and the quality control of traditional Chinese medicine preparations, can solve the problems of large interference of impurities, difficulty in identification, lack of specificity, etc., and achieve high stability and effective quality control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0041] Experimental Example 1 Study on Quality Control of Gongyanping Capsule Raw Materials (Materials)
[0042] Diren is included in the "Quality Standards for Traditional Chinese Medicinal Materials and Ethnic Medicinal Materials in Guizhou Province" (DB52 / YC001~420-2003), and the medicinal materials of Liangmianzhen and Angelica are included in the "Chinese Pharmacopoeia" 2000 edition. Stone is a collection of "Chinese Pharmacopoeia" 1977 edition.
[0043] 1 Diren According to the literature reports and experiments on the chemical components contained in the medicinal materials of Diren, we determined the content of gallic acid in the medicinal materials of Diren, and studied the method of content determination.
[0044] 1.1 Instrument High performance liquid chromatography system (Shimadzu LC-10Avp), including 2 LC-10ATvp pumps; SPD-10Avp UV detector, 7725i manual injection valve, TC-100 column thermostat (Tianjin Turner Technology Co., Ltd.) , WML chromatography workstat...
experiment example 2
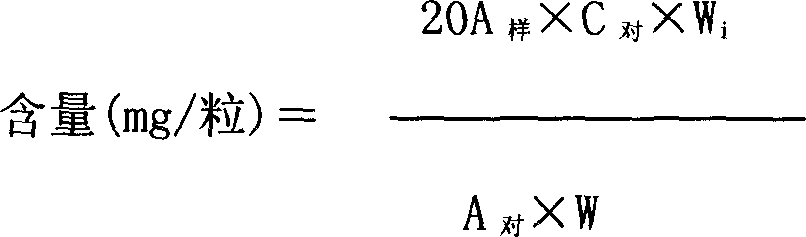
[0087] Experimental Example 2 Study on the Quality Control of Gongyanping Capsules Finished Products
[0088] 1 Name This product is "Gongyanping Tablets" (Standard No.: WS) 3 -B-3274-98) obtained by modifying capsules, according to the relevant regulations on drug naming, adopting the original species name plus dosage form and named it "Gongyanping Capsule". Chinese Pinyin: Gongyanping Jiaonang.
[0089] 2 Prescription This prescription is composed of Diren, Liangmianzhen, Angelica, Wuzhi Maotao, and Chuanposhi five flavors. The prescription of Gongyanping Tablets contains 1000g of crude drug in total. It is made into 1000 tablets. The daily dosage is 12 tablets, which is equivalent to 12g of crude drug. Yanping Capsules is made of 2000g crude drug into 1000 capsules, taking 6 capsules per day, which is equivalent to taking 12g of crude drug per day, which is the same amount of total medicinal materials as Gongyanping Tablets daily. Therefore, the prescription quantity is D...
Embodiment 1
[0161] The quality control of embodiment 1 Gongyanping Capsules
[0162] [Prescription] Diren 900g, Liangmianzhen 340g, Angelica 280g, Wuzhi Maotao 200g, Chuanposhi 280g
[0163] [Preparation method] The above five flavors, add 10 times the amount of water to decoct twice, each time for 2 hours, filter, and combine the filtrates. Concentrate to a relative density of 1.25 (55-60°C). Add ethanol and stir evenly to make the alcohol content reach 50%, let stand for 24 hours, and filter. Recover ethanol from the filtrate, concentrate to a thick paste, add an appropriate amount of starch, mix, and dry under reduced pressure. Capsules, made into 1000 capsules, ready to use.
[0164] [Properties] This product is a capsule, the contents are brown to tan particles; slightly gas, bitter, sour, slightly astringent.
[0165] [Identification] (1) Take 1g of the content of this product, add 25ml of 70% ethanol, heat and reflux for 1 hour, let cool, filter, evaporate the filtrate to nearl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com