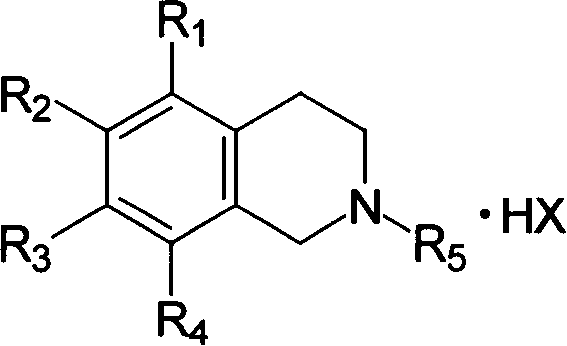
Tetra hydro iso quinoline compounds possessing anti breed and anti fungus activity and its salt
A tetrahydroisoquinoline, anti-fertility technology, applied in the field of medicine, can solve the problems of affecting clinical treatment, high toxicity of drugs, limiting clinical application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
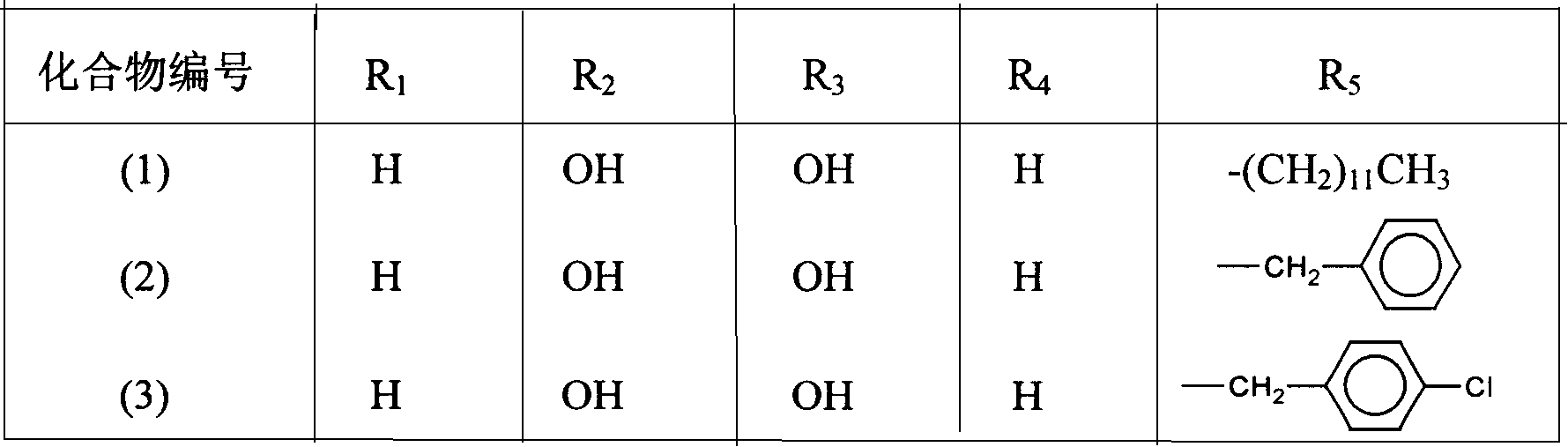
[0036] The preparation of embodiment 1,2-dodecyl-6,7-dihydroxyl-1,2,3,4-tetrahydroisoquinoline hydrobromide (compound 1 in table 1)
[0037] Add 20ml of 3,4-dimethoxy-phenylethylamine and 3.60g of paraformaldehyde to absolute ethanol, stir and react at room temperature until the paraformaldehyde is completely reacted, adjust the pH of the solution to 2 with dilute hydrochloric acid, and heat to reflux for 4 hours , cooled, precipitated solid, filtered, added the resulting solid to 10% NaOH solution, and then added CH 2 Cl 2 100ml, shake, separate CH 2 Cl 2 layer, anhydrous K 2 CO 3 Dry, filter, and remove the solvent under reduced pressure to obtain 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline as a pale yellow solid.
[0038] Take the compound 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline 1g and 0.6g dodecane bromide obtained above, K 2 CO 3 1g was dissolved in 30ml of absolute ethanol, stirred and refluxed for 8h, allowed to cool, filtered, and the solvent was evaporat...
Embodiment 2
[0056] The preparation of embodiment 2,2-dodecyl-6,7-diacetoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (compound 4 in table 1)
[0057] Get 3.5g 6,7-dihydroxyl-2-dodecyl-1,2,3,4-tetrahydroisoquinoline hydrobromide and join in 5ml pyridine and 30ml dichloromethane solution, ice bath cooling, Slowly add 8ml of acetic anhydride dropwise under stirring, after the addition is complete, stir at room temperature for 4h, then heat to reflux for 3h. Let it cool, and the reaction solution was washed with NaHCO 3 Aqueous solution and water wash. Na for reaction solution 2 CO 3 After drying, the solvent was evaporated to obtain a yellow solid. The solid was dissolved in 30ml of absolute ethanol, and HCl gas was introduced to saturation under an ice bath, and the solid was precipitated after standing, filtered, and dried to obtain 6,7-diacetoxy-2-dodecyl-1,2, 3,4-tetrahydroisoquinoline hydrochloride.
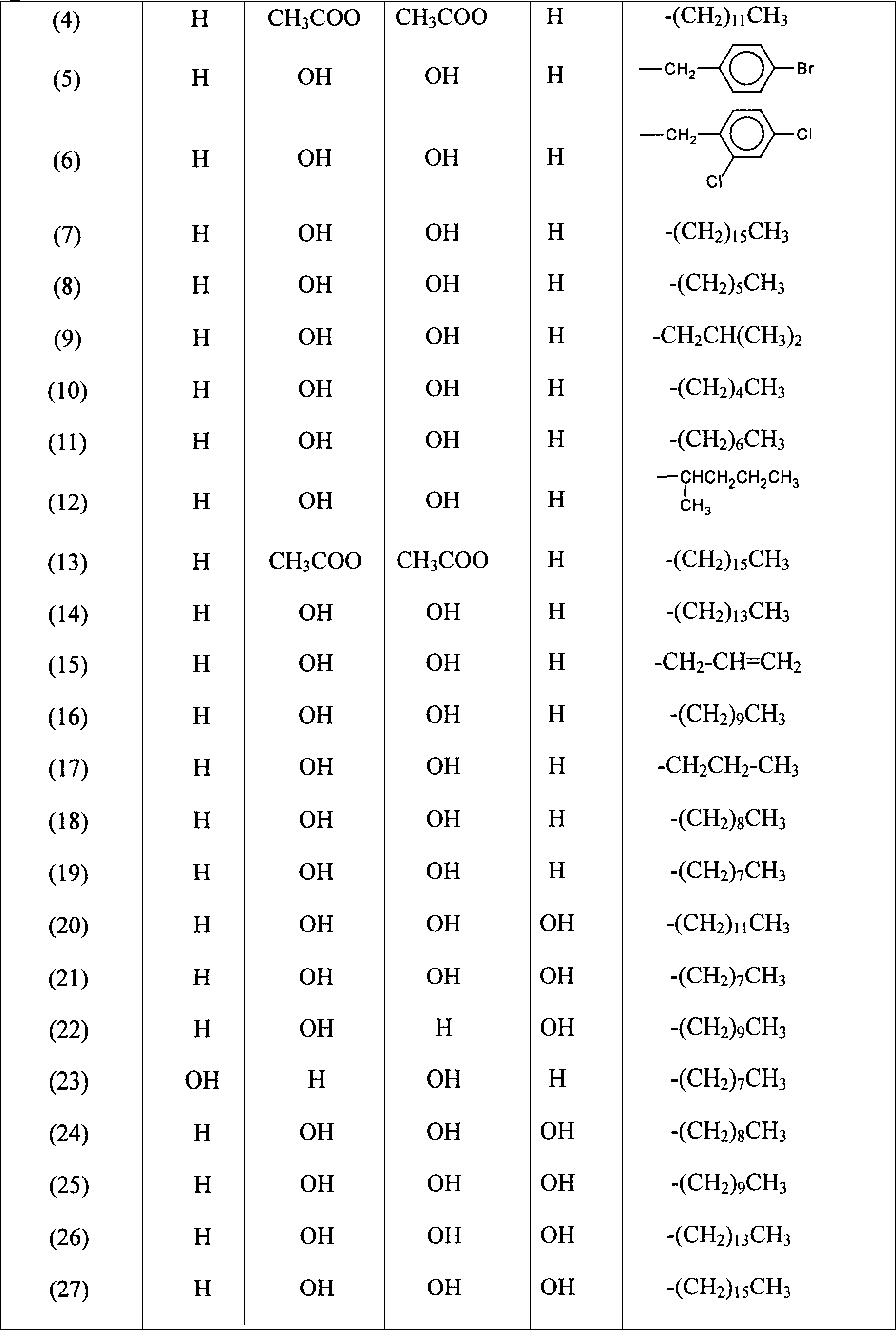
[0058] The following compounds are prepared by the method of Example 2:
[0...
Embodiment 3
[0060] Embodiment 3, the preparation of 2-decyl-6,8-dihydroxyl-1,2,3,4-tetrahydroisoquinoline hydrochloride (compound 22 in table 1)
[0061] 18 g of 3,5-dihydroxyphenethylamine was reacted with 3.6 g of paraformaldehyde, and the method was the same as in Example 1 to obtain 6,8-dihydroxy-1,2,3,4-tetrahydroisoquinoline.
[0062] The intermediate 6.1g prepared above, bromodecane 6.2g, anhydrous K 2 CO 3 5.6 g and 150 ml of acetonitrile, stirred at room temperature for 16 hours, removed the solvent under reduced pressure, dissolved in ethyl acetate, washed with water, dried, and passed through HCl gas to obtain 2-decyl-6,8-dihydroxy-1,2,3 , 4-tetrahydroisoquinoline hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com