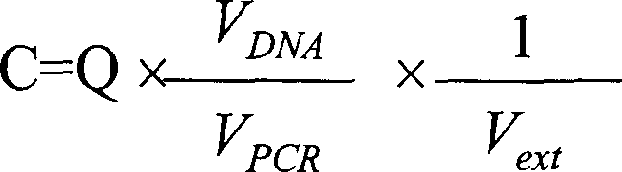Screening of preinvasive carcinoma, carcinoma in situ and carcinoma focus by using DNA of circulating epstein-barr virus
A technology for precancer and cancer, which is applied in the field of screening of precancer, carcinoma in situ and cancer lesions by using circulating ibovirus DNA, which can solve the problems such as hindering the immune surveillance of tumor antigens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0047] Materials and methods
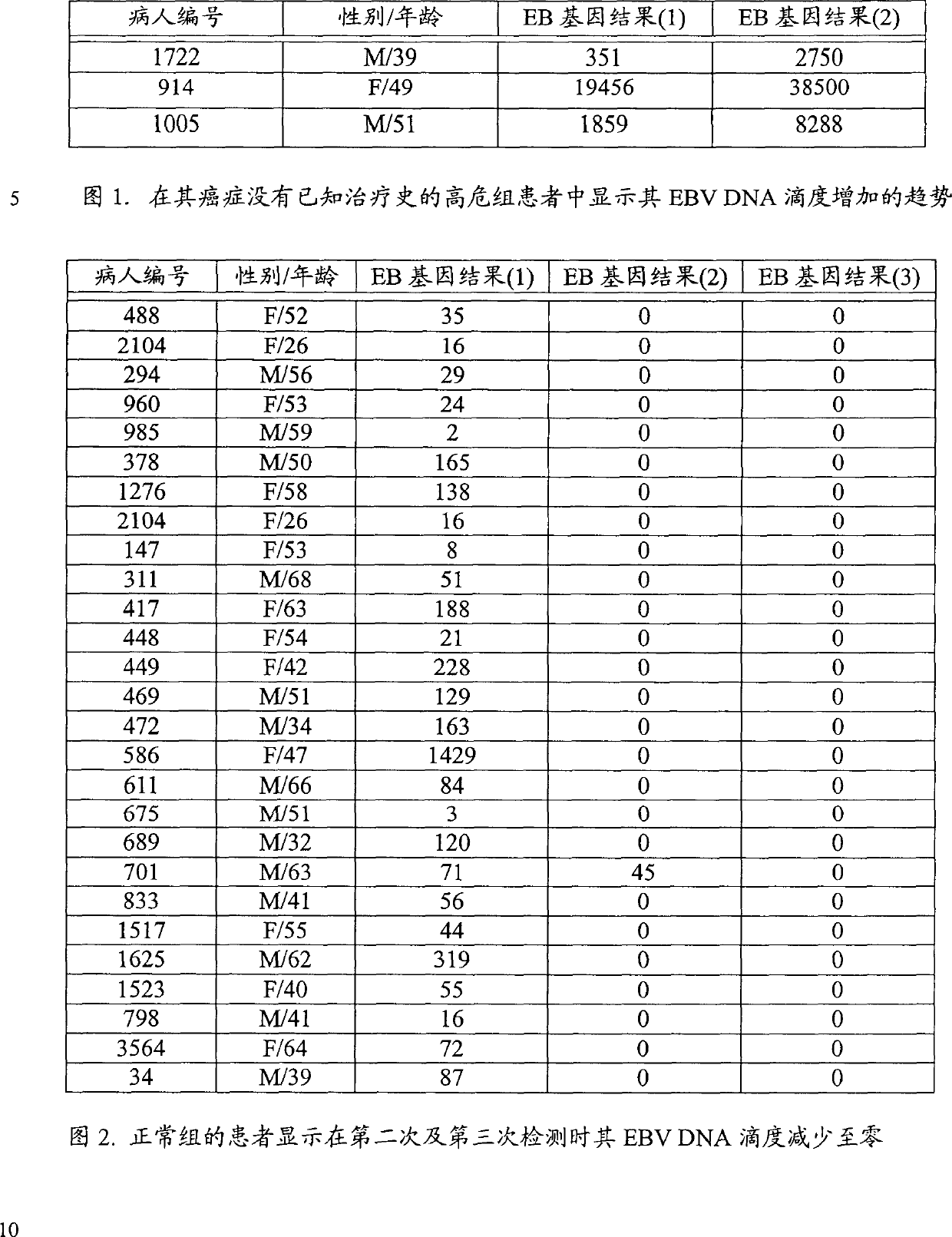
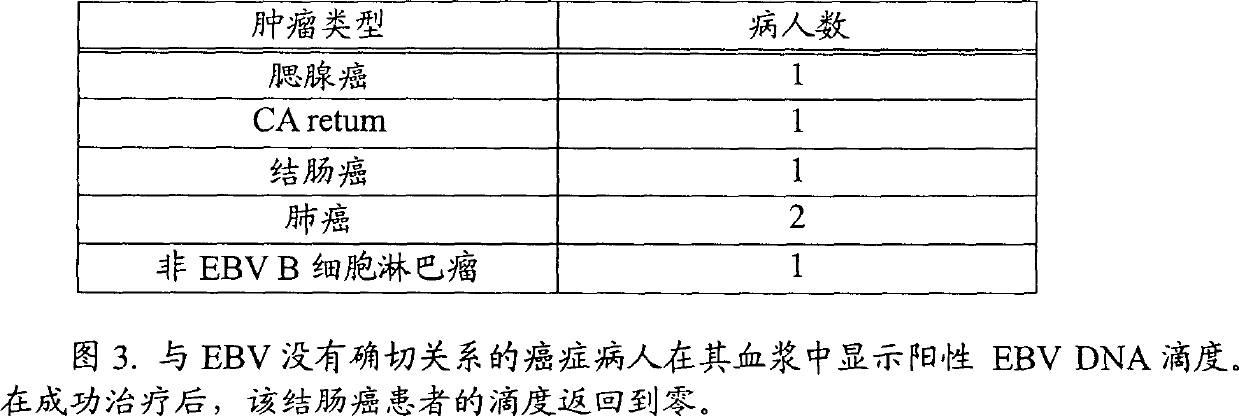
[0048] Blood samples from 132 high-risk cancer patients (Group A) and 3987 approximately normal individuals (Group B) have been sent to our laboratory in Hong Kong for the determination of circulating EBV DNA.
[0049] DNA Extraction from Plasma Samples Peripheral blood (5 ml) was collected from each subject and injected into EDTA tubes for separation of plasma. Blood samples were centrifuged at 1600 x g, and the plasma was carefully separated from the EDTA-containing tubes and transferred into blank polypropylene tubes. This sample was stored at -20°C until further manipulation. DNA was extracted from plasma samples using the QIAamp Blood Kit (Qiagen, Hilden, Germany) using the blood and body fluids procedure according to the manufacturer's recommendations (2). DNA extraction was performed using plasma samples (130-800 μl / column). Record the exact amount used to calculate the target DNA concentration. The volume of fina...
Embodiment II
[0066] Materials and methods
[0067] The percentage of CD4+CD25+ T cells in the peripheral blood of 20 patients with positive EBV DNA titers (group C) was compared with the EBV DNA-negative control group.
[0068] Peripheral blood from these 20 patients was obtained at the time of blood collection, injected into a Vacutainer tube containing heparin, diluted 2:1 with 1×Dulbecco's phosphate buffer (Mediatech) without calcium and magnesium, and then incubated at room temperature on poly Glycosomes (ficoll) were separated by centrifugation at 1000×g for 20 minutes on a density gradient. Peripheral blood mononuclear cells were collected, washed and cryopreserved in RPMI1640 containing 20% fetal bovine serum and 10% DMSO for future use.
[0069] Cell types were determined using a four-color hemocytometer and flow cytometric analysis using activity markers of anti-CD3-PerCP, anti-CD4-APC or anti-CD8-FIT, anti-CD25-PE. Briefly, cells were incubated for 30 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com