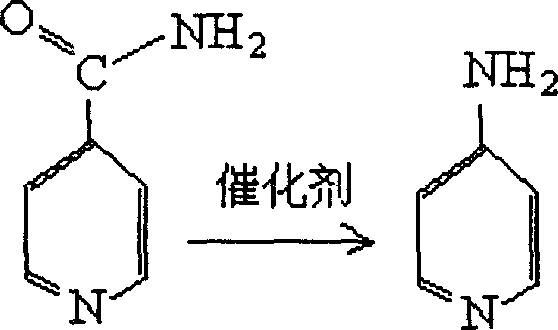
4-aminopyridine preparation method
A technology of aminopyridine and isonicotinamide, which is applied in the field of preparation of 4-aminopyridine, can solve the problem that the yield of Hofmann degradation reaction is not very high, and achieve the effects of low price, mild reaction conditions and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Weigh 2.54g (0.01mol) of commercially available analytically pure iodine and put it into a 500ml three-necked bottle, add 18g (1mol) of deionized water to dissolve it, and then put the three-necked bottle into ice at 0-5°C In the water bath, add 22g (0.55mol) of commercially available analytically pure sodium hydroxide, add 90g (5mol) of deionized water under stirring conditions to make it dissolve, then slowly add dropwise commercially available analytically pure bromine 39.95g (0.25mol ), after fully stirring evenly, it is the prepared catalyst.
[0017] (2) Weigh 26.87g (0.22mol) of commercially available chemically pure isonicotinamide, add it to the above-mentioned three-necked flask containing the prepared catalyst, keep the reaction temperature at 0-5°C, stir for 45min, then slowly increase Reaction temperature to 80°C, and keep it for 50min, then, add 1N dilute hydrochloric acid solution to strong acidity (pH value is 1~2), cool down to room temperature unde...
Embodiment 2
[0020] (1) Weigh 1.66g (0.01mol) of commercially available analytically pure potassium iodide into a 500ml three-necked bottle, add 27g (1.5mol) of deionized water to dissolve it, and then put the three-necked bottle into a 0-5°C In the ice-water bath, add commercially available analytically pure potassium hydroxide 28.06g (0.50mol), add deionized water 72g (4mol) under stirring condition and make it dissolve, then slowly add dropwise commercially available analytically pure bromine 47.94g ( 0.30mol), after fully stirring evenly, it is the prepared catalyst.
[0021] (2) Weigh 30.53g (0.25mol) of commercially available chemically pure isonicotinamide, add it to the three-necked flask containing the prepared catalyst, keep the reaction temperature at 0-5°C, stir for 50min, and then slowly increase Reaction temperature to 75°C, and keep it for 55min, then, add 1N dilute hydrochloric acid solution to strong acidity (pH value is 1~2), cool down to room temperature while stirring, ...
Embodiment 3
[0024] (1) Take commercially available analytically pure sodium iodide 1.50g (0.01mol) and put it into a 500ml there-necked bottle, add 18g (1mol) of deionized water and 22ml of deionized water to dissolve it, and then put the there-necked bottle into Add 28g (0.70mol) of commercially available analytically pure sodium hydroxide to an ice-water bath at 0-5°C, add 72g (4mol) of deionized water under stirring conditions to dissolve it, then slowly add dropwise commercially available analytically pure sodium hydroxide 43.15g (0.27mol) of bromine, after fully stirring evenly, is the prepared catalyst.
[0025] (2) Weigh 25.65g (0.21mol) of commercially available chemically pure isonicotinamide, add it to the three-necked flask containing the prepared catalyst, keep the reaction temperature at 0-5°C, stir for 45min, and then slowly increase Reaction temperature to 70°C, and keep it for 60min, then, add 1N dilute hydrochloric acid solution to strong acidity (pH value is 1~2), cool d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com