Fasudic hydrochloride oral formulation
A preparation technology of fasudil hydrochloride, which is applied in the direction of pill delivery, medical preparations containing active ingredients, cardiovascular system diseases, etc., can solve the problems of complicated preparation process, difficult preparation production, inconvenient use, etc., and achieve the goal of using convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
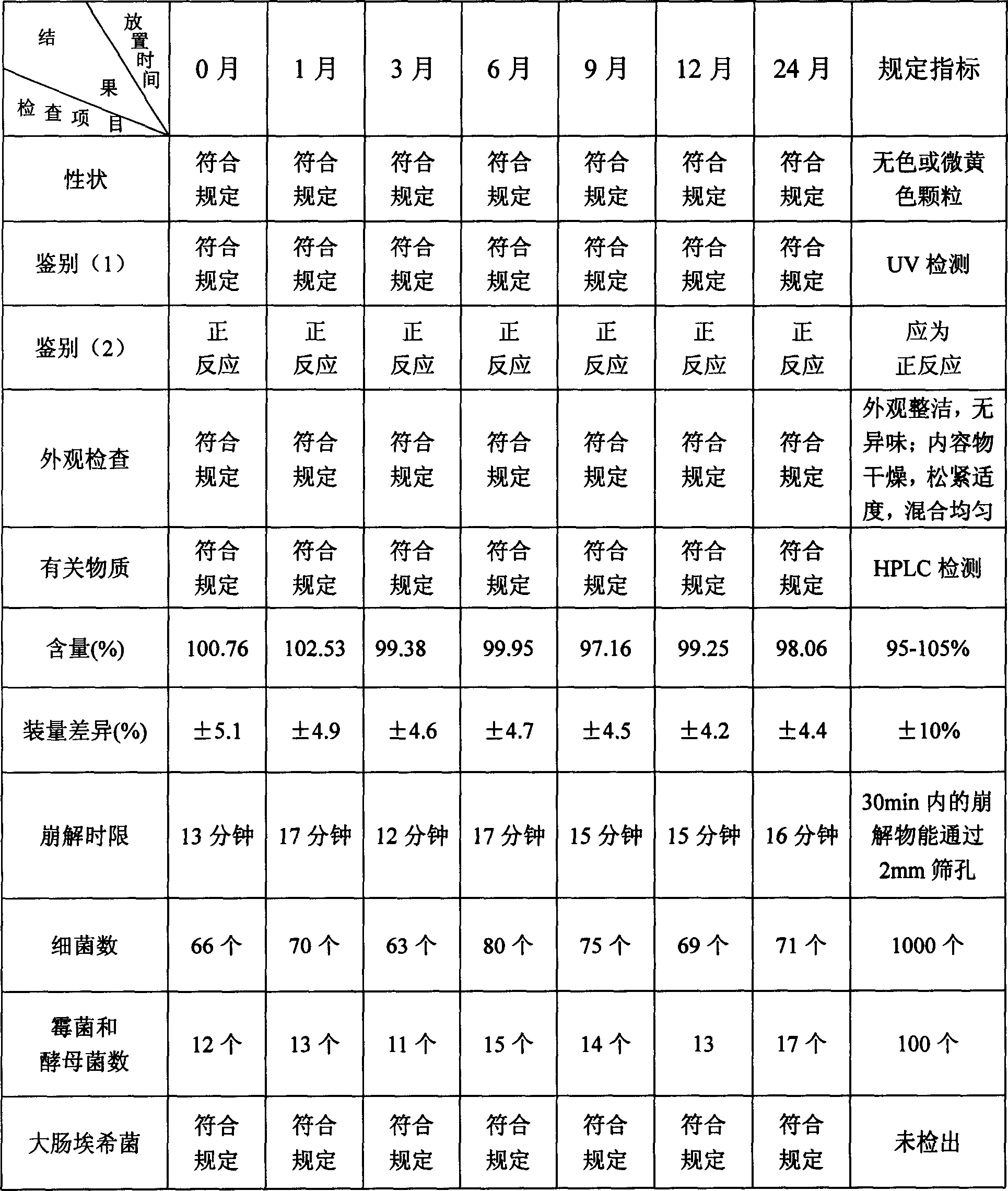
[0149] Example 1 tablet
[0150] Fasudil Hydrochloride 80g
[0151] Lactose 150g
[0152] Hydroxypropyl Cellulose (HPC) 4g
[0153] water 560ml
[0154] Opadry AMB (Opadry) 12.5g
[0155] Sodium Carboxymethyl Starch (CMS Na) 12g
[0157] Titanium dioxide 6g
[0158] Preparation:
[0159] Dissolve 80g of fasudil hydrochloride in 250ml of water to prepare an aqueous solution of fasudil hydrochloride. Dissolve 4g of hydroxypropyl cellulose in 150ml of water to form an aqueous solution of hydroxypropyl cellulose. Adopt the fluidized boiling granulation method, put 150g lactose and 12g carboxymethyl starch sodium into the fluidization chamber screen and be boiling state in the hot air of 60 ℃, spray into fasudil hydrochloride aqueous solution, the lactose and the boiling state The mixture of sodium carboxymethyl starch and the main ingredient starts to coalesce into granules, and after repeated spraying and drying, granules are obtained. Add 4 g of...
Embodiment 2
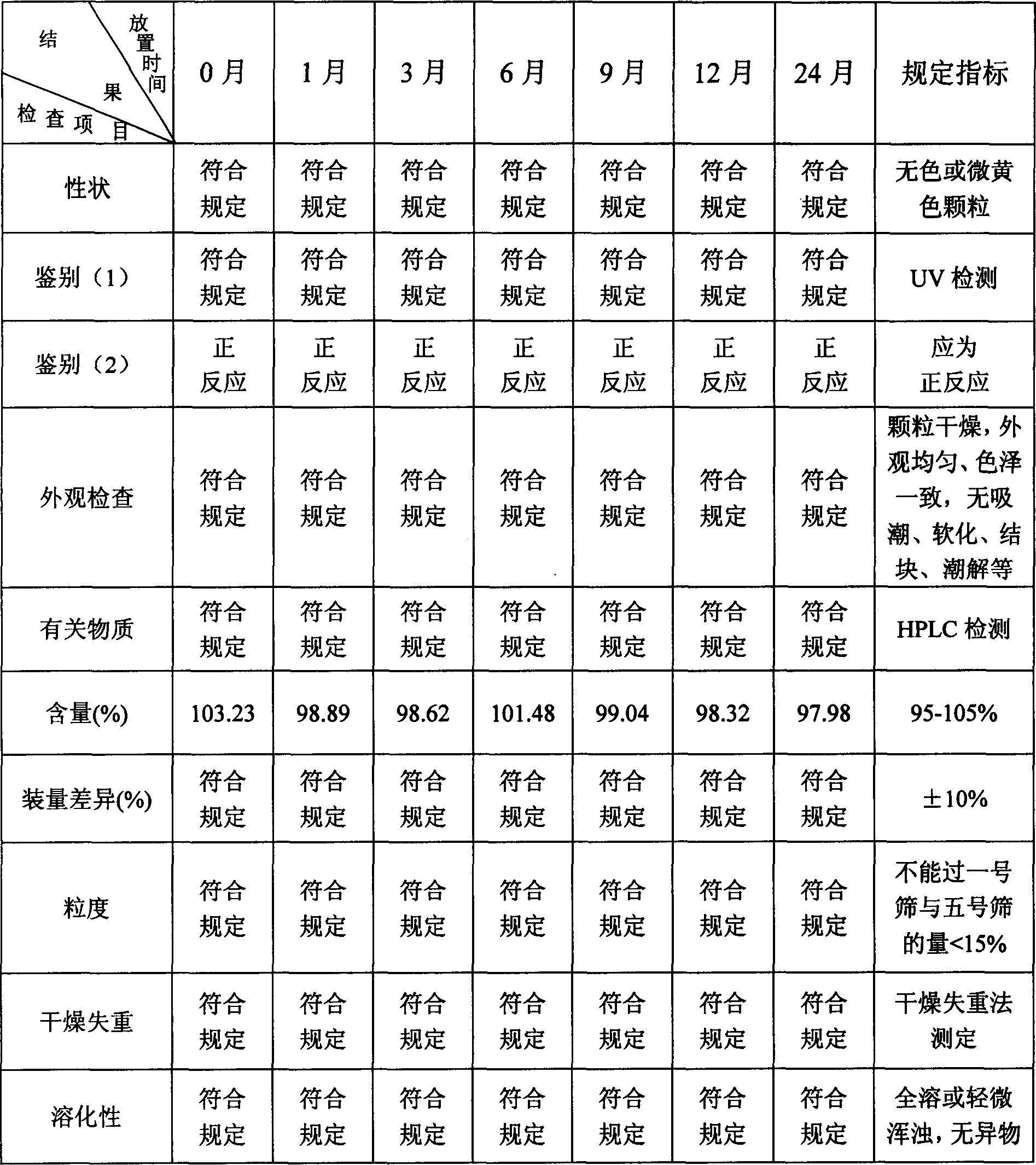
[0160] Embodiment 2 Granules
[0161] Fasudil Hydrochloride 80g
[0162] Lactose 150g
[0163] Hydroxypropyl Cellulose (HPC) 4g
[0164] water 560ml
[0165] Sodium Carboxymethyl Starch (CMS Na) 12g
[0166] The preparation method is:
[0167] Dissolve 80g of fasudil hydrochloride in 250ml of water to prepare an aqueous solution of fasudil hydrochloride. Dissolve 4g of hydroxypropyl cellulose in 150ml of water to form an aqueous solution of hydroxypropyl cellulose. Adopt the fluidized boiling granulation method, put 150g lactose and 12g carboxymethyl starch sodium into the fluidization chamber screen and be boiling state in the hot air of 60 ℃, spray into fasudil hydrochloride aqueous solution, the lactose and the boiling state The mixture of sodium carboxymethyl starch and the main ingredient starts to coalesce into granules, and after repeated spraying and drying, granules are obtained.
Embodiment 3
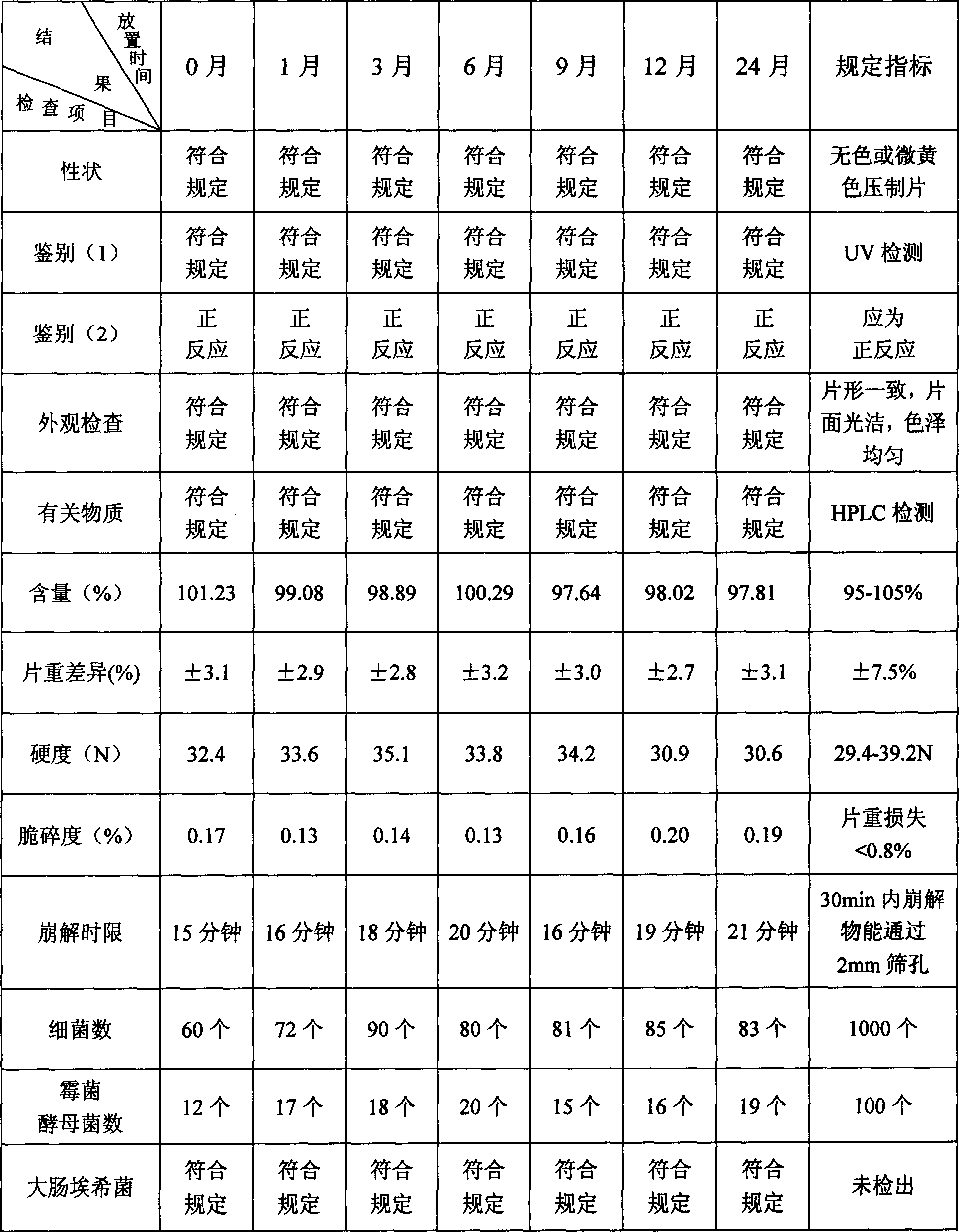
[0168] Embodiment 3 capsules
[0169] Fasudil Hydrochloride 80g
[0170] Lactose 150g
[0171] Hydroxypropyl Cellulose (HPC) 8g
[0172] water 560ml
[0173] Sodium Carboxymethyl Starch (CMS Na) 12g
[0175] Ethanol 80ml
[0176] The preparation method is:
[0177] Dissolve 80g of fasudil hydrochloride in 250ml of water to prepare an aqueous solution of fasudil hydrochloride. Dissolve 4g of hydroxypropyl cellulose in 150ml of water to form an aqueous solution of hydroxypropyl cellulose. Adopt the fluidized boiling granulation method, put 150g lactose and 12g carboxymethyl starch sodium into the fluidization chamber screen and be boiling state in the hot air of 60 ℃, spray into fasudil hydrochloride aqueous solution, the lactose and the boiling state The mixture of sodium carboxymethyl starch and the main ingredient starts to coalesce into granules, and after repeated spraying and drying, granules are obtained. After granulation, the granules an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com