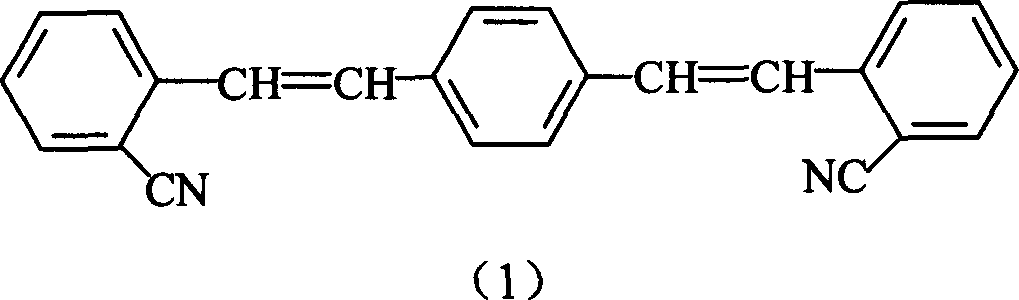
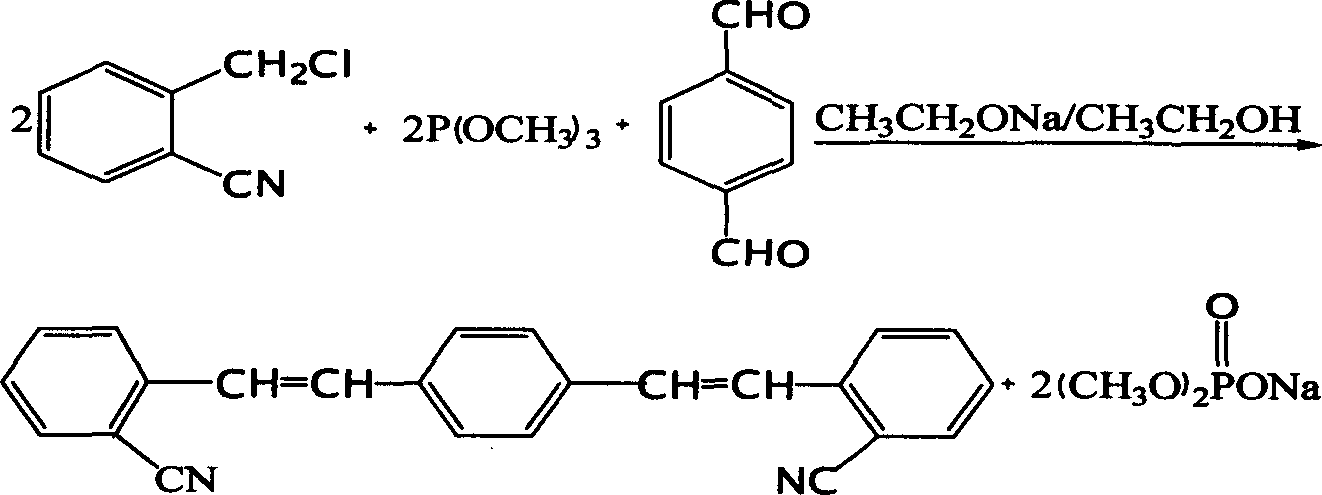
Production of 1,4-bis(O-styryl)
A technology of cyanostyrene and o-cyanobenzyl, applied in 1 field, can solve the problems of low product purity and yield, cumbersome operation, high energy consumption, etc., and achieve the effects of simple operation, low energy consumption, energy consumption and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 16g of commercially available o-cyanobenzyl chloride (99wt%) into a 250mL four-neck flask with a condenser, stir and heat up to 100°C, add 13g of trimethyl phosphite (98wt%) within 2.5 hours After the addition, the temperature was raised to 130°C, and the stirring reaction was continued for 2 hours to remove the methyl chloride gas, then the reaction liquid was cooled to 28°C, and 6.3g of terephthalaldehyde and 10g of N,N-dimethylformamide (DMF) were added, Stir evenly, add 54g of sodium ethylate ethanol solution (16wt%) dropwise within two hours at a temperature of 30°C, react for 2 hours, filter to obtain the crude product, then wash with ethanol and water in turn, filter and dry to obtain yellow-green crystals The product has a melting point of 230-231° C. and a reaction yield of 87%.
Embodiment 2
[0024] Add 62g of commercially available o-cyanobenzyl chloride (99wt%) into a 500mL four-neck flask with a condenser, stir and heat up to 110°C, add 51g trimethyl phosphite (98wt%) within 2.5 hours After the addition, the temperature was raised to 120°C, and the stirring reaction was continued for 3 hours to remove the methyl chloride gas, then the reaction solution was cooled to 20°C, 25g of terephthalaldehyde and 50g of DMF were added, and 215g of sodium ethylate ethanolic solution was added dropwise within two hours ( 10wt%), the reaction time was continued for 2 hours, the crude product was obtained by filtration, washed with ethanol and water, and dried to obtain a yellow-green crystalline product with a melting point of 229-231° C. and a yield of 82%.
Embodiment 3
[0026] According to the feeding ratio and method of Example 2, 50 g of dimethylformamide solvent was changed to 50 g of tetrahydrofuran. The crude product was washed with ethanol and water, and dried to obtain a yellow-green crystal product with a melting point of 228-230° C. and a yield of 73%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com