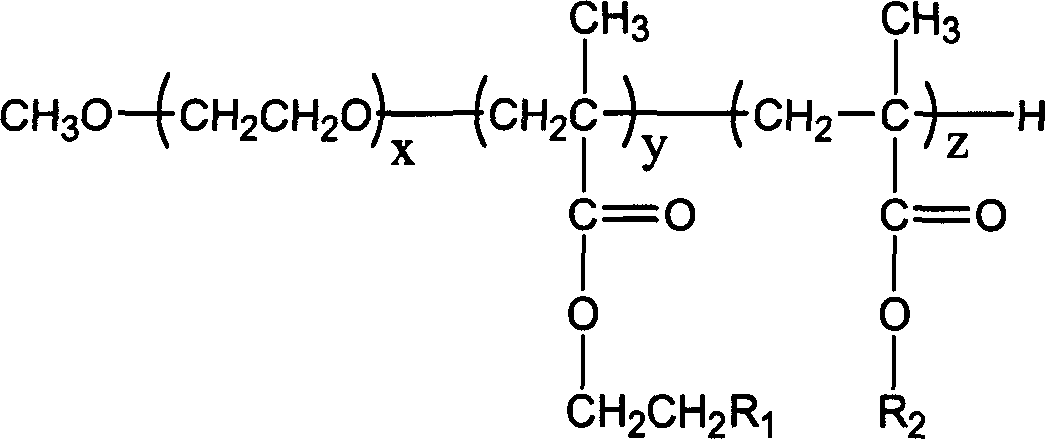
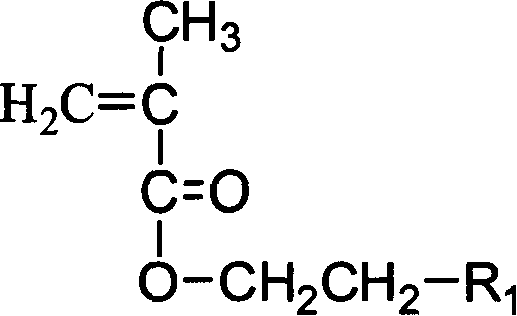
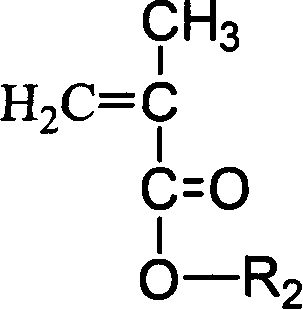
Amphipathic fluoride block copolymer and production thereof
A block copolymer and amphiphilic technology, applied in the field of amphiphilic fluorine-containing block copolymer and its preparation, can solve the problems of influence of polymer properties, difficult to remove, etc., and achieve fast reaction speed, easy purification and improvement Effect of temperature/pH responsive performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: MePEG-b-PDMAEMA-b-POFPMA and its preparation
[0044] (1) Preparation of KH: Put the stirring rotor into a dry reaction bottle in advance, and plug it tightly with a reversible rubber stopper. Then use a needle and a latex tube to connect with a vacuum pump, and fill it with high-purity argon gas while vacuuming, and repeat the operation three times. After moving a certain amount of KH into the reaction bottle, inject 5ml of dry THF with a dry syringe, stir and wash, and suck out the THF containing mineral oil with a syringe after resting, repeat this three times, and finally dry the residual THF solution with high-purity argon. Accurately weigh the amount of KH in the reaction flask (0.2-0.3 g, about 5.0-7.5 mmol) by the subtraction method.
[0045] (2) Preparation of initiator: inject 15-30ml of THF into the reaction bottle, place the reaction bottle in a constant temperature oil bath at 20°C, and inject dimethyl sulfoxide (DMSO) with KH and other substanc...
Embodiment 2
[0047] Example 2: MePEG-b-PDMAEMA-b-POFPMA and its preparation
[0048] (1) Preparation of KH: same as Example 1.
[0049] (2) Preparation of initiator: same as Example 1.
[0050] (3) Polymerization reaction: Move the reaction bottle into a constant temperature oil bath at 40°C and add the first monomer 2-(dimethylamino)ethyl methacrylate (DMAEMA) with a dry syringe to control the reaction between the first monomer and the initiator. The molar ratio of the agent is 30:1, and the first monomer DMAEMA monomer equivalent to 30 times the amount of the initiator is injected to carry out the polymerization reaction for 1~1.5h, and the molar ratio of the second monomer and the initiator is controlled to be 6:1, Use a dry syringe to add the second monomer methacrylic acid-(2,2,3,3,4,4,5,5-octafluoro)pentyl ester (OFPMA) equivalent to 6 times the amount of the initiator, and react 1~ 1.5h, and finally terminate the reaction with dry methanol. The reacted polymer is subjected to rot...
Embodiment 3
[0051] Example three: MePEG-b-PDMAEMA-b-POFPMA and its preparation
[0052] (1) Preparation of KH: same as Example 1.
[0053] (2) Preparation of initiator: same as Example 1.
[0054] (3) Polymerization reaction: Move the reaction bottle into a constant temperature oil bath at 40°C and add the first monomer 2-(dimethylamino)ethyl methacrylate (DMAEMA) with a dry syringe to control the reaction between the first monomer and the initiator. The molar ratio of the agent is 50:1, and the first monomer DMAEMA monomer equivalent to 50 times the amount of the initiator is injected to carry out the polymerization reaction for 1~1.5h, and the molar ratio of the second monomer and the initiator is controlled to be 6:1, Then add the second monomer methacrylic acid-(2,2,3,3,4,4,5,5-octafluoro)pentyl ester (OFPMA) equivalent to 6 times the amount of initiator with a dry syringe, and react 1 ~1.5h, and finally terminate the reaction with dry methanol. The reacted polymer is subjected to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com