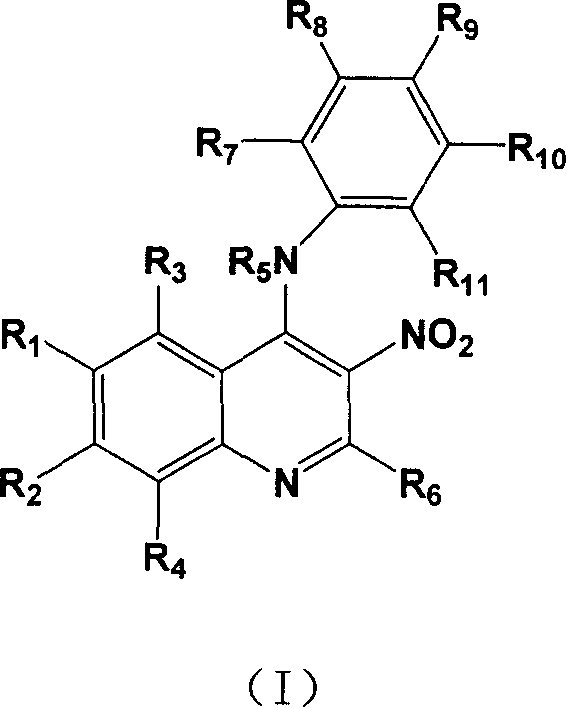
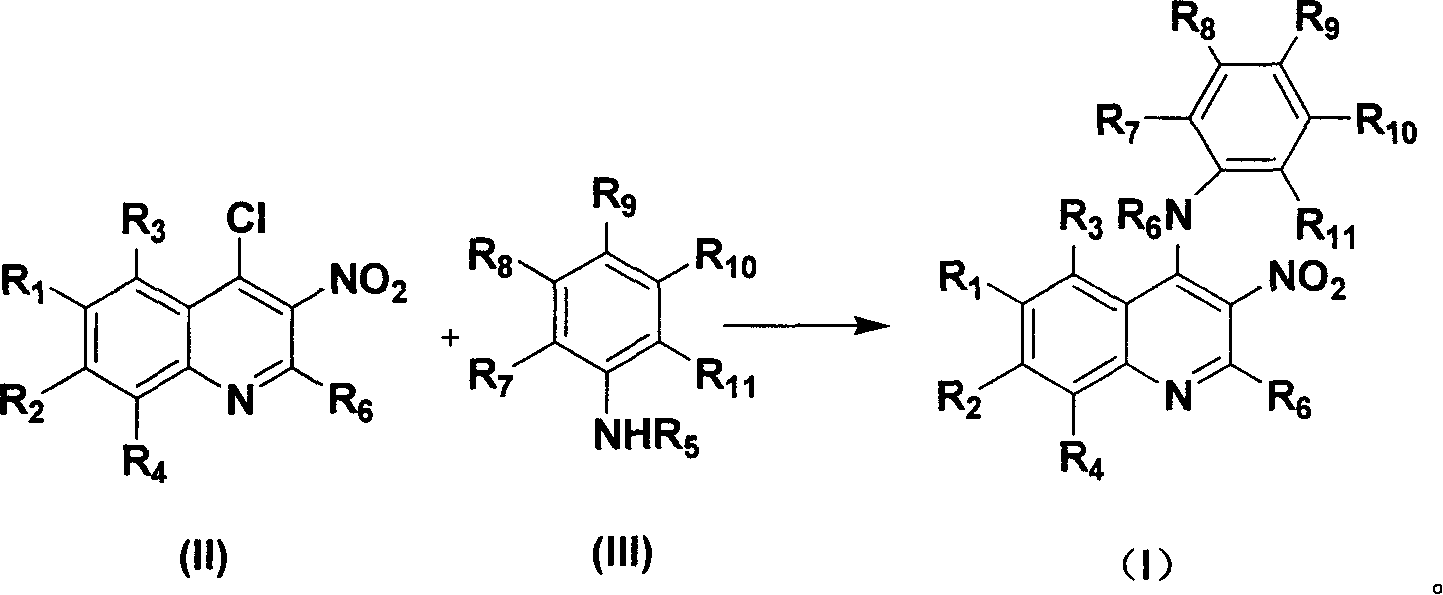
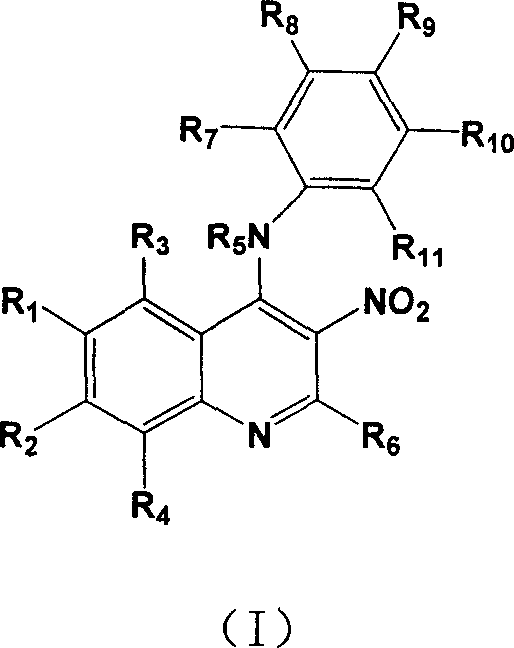
4-Substituting anilino-3-nitroquinoline compounds, prepn. method and use thereof
A technology of nitroquinoline and anilino, applied in the field of preparation of such compounds, can solve the problems of unknown toxicity of 4-anilinoquinazoline compounds, and achieve the effects of easy preparation and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of 6-benzyloxy-7-methoxy-4-(3-ethynylanilino)-3-nitroquinoline (F-1)
[0039] Dissolve isovanillin (9g, 0.059mol) in 40ml of ethanol, add 8.1g of anhydrous sodium carbonate, add distilled benzyl chloride (11.4g, 0.09mol) dropwise, heat to reflux for 5 hours, and spot the plate until the reaction completely. The reaction solution was filtered while it was hot, and the filtrate was evaporated to dryness to obtain an oil, which was solidified at room temperature and recrystallized with ethanol to obtain 10.3 g of 4-benzyloxy-3-methoxybenzaldehyde as a white solid, with a yield of 76%. . h 1 NMR (CDCl 3 ): 9.8 (s, 1H, CHO), 7.3-7.5 (m, 5H, Ph-H), 7.25-7.28 (m, 2H, Ph-H), 7.0 (s, 1H, Ph-H), 5.25 ( s, 2H, PhCH 2 O), 4.0(s, 3H, CH 3 O).
[0040] In an ice bath at 0°C, 4-benzyloxy-3-methoxybenzaldehyde (10 g, 0.041 mol) was slowly added to 40 ml of concentrated nitric acid, and stirring was continued at 15°C for 40 min after the addition was complet...
Embodiment 2
[0048] Example 2 Preparation of 6-benzyloxy-7-methoxy-4-(3-chloroanilino)-3-nitroquinoline (F-2)
[0049] 6-Benzyloxy-7-methoxy-4-chloro-3-nitroquinoline (1.0 g, 2.9 mmol) and 3-chloroaniline (368 mg, 2.9 mmol) were heated in DMF (20 ml) to 100 ℃. After stirring for 24 hours, the solvent was evaporated under reduced pressure, and the residue was recrystallized from methanol to obtain 1.14 g of 6-benzyloxy-7-methoxy-4-(3-chlorophenylamino)-3-nitroquine as a yellow solid Phenyl (F-2), the yield is 90%. h 1 NMR (DMSO): 9.89 (s, 1H, quinoline-2-H), 7.19-7.68 (m, 9H, Ph-H), 5.20 (s, 2H, CH 2 Ph), 4.0(s, 3H, OCH 3 ).
Embodiment 3
[0050] Example 3 Preparation of 6-benzyloxy-7-methoxy-4-(3-bromoanilino)-3-nitroquinoline (F-3)
[0051] 6-Benzyloxy-7-methoxy-4-chloro-3-nitroquinoline (1.0g, 2.9mmol) and 3-bromoaniline (500mg, 2.9mmol) in DMF (20ml) were heated to 100 ℃. After stirring for 24 hours, the solvent was evaporated under reduced pressure, and the residue was recrystallized from methanol to obtain 1.26 g of 6-benzyloxy-7-methoxy-4-(3-bromophenylamino)-3-nitroquine as a yellow solid Phenyl (F-3), yield 91%. h 1 NMR (DMSO): 9.77 (s, 1H, quinoline-2-H), 7.19-7.68 (m, 4H, Ph-H), 5.20 (s, 2H, CH 2 Ph), 4.0(s, 3H, OCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com