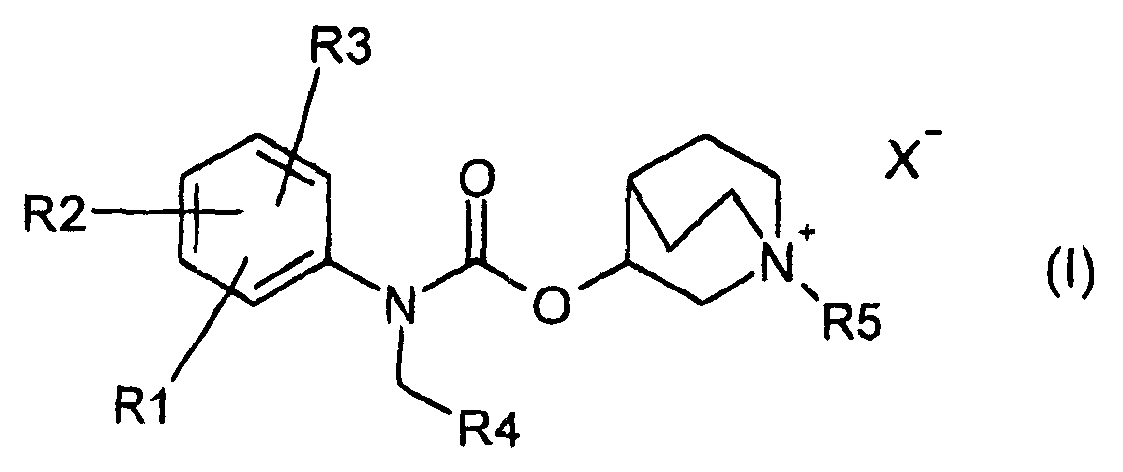
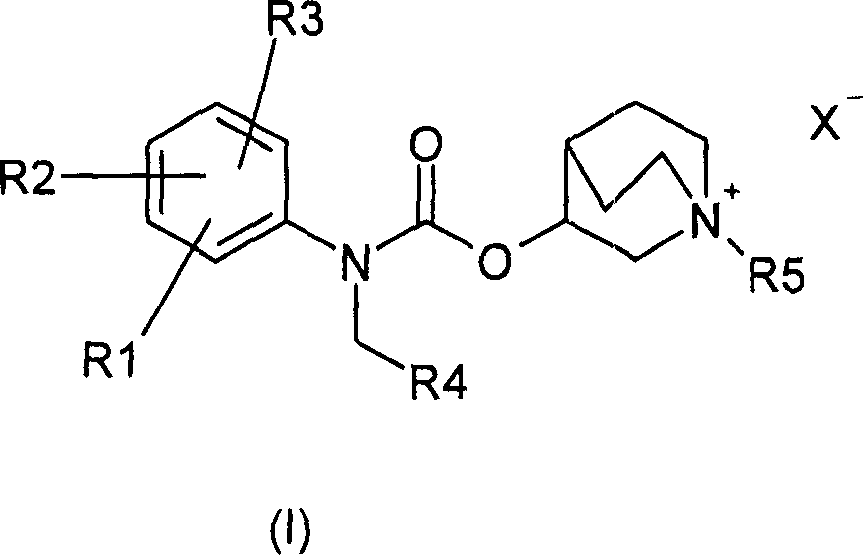
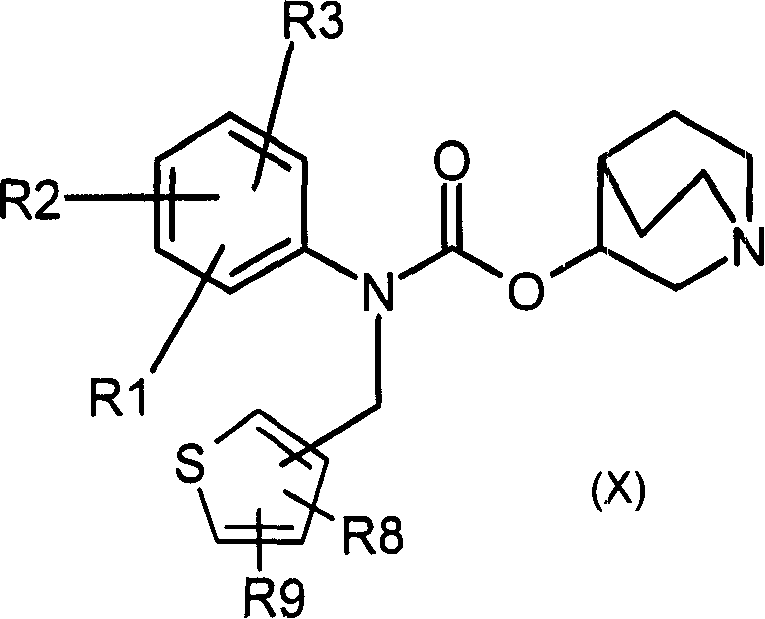
1- alkyl-1-azoniabicyclo (2.2.2) octane carbamate derivatives and their use as muscarinic receptor antagonists
A technology of alkyl and alkoxy, applied in the field of 1-alkyl-1-azonium bicyclo[2.2.2]octane carbamate derivatives and their use as muscarinic receptor antagonists , can solve the problem of decreased M2 receptor affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Example 1: (R)-3-(benzylphenylcarbamoyloxy)-1-cyclopropyl-1-azonium bicyclo[2.2.2]octane bromide
[0149] 200 mg (0.59 mmol) of intermediate 3 and 0.47 mL of bromocyclopropane (0.59 mmol) were mixed in 5 mL of acetonitrile / chloroform (2:3). The resulting solution was refluxed for 12 hours. The solvent was evaporated and the residue was subjected to column chromatography [SiO 2 , eluent: dichloromethane-methanol (20:1)] to give 130 mg (47%) of the title compound as a hygroscopic white solid. 1 H-NMR (CDCl 3 ): 7.27(m, 10H), 4.87(m, 2H), 4.80(m, 1H), 3.18(ddd, 1H), 3.01(m, 1H), 2.80-2.50(m, 5H), 2.23(m, 1H), 1.98(m, 2H), 1.65-1.18(m, 6H).
[0150] Synthesize the following compounds according to Example 1:
Embodiment 2
[0151] Example 2: Chloride (R)-3-(benzylphenylcarbamoyloxy)-1-(2-chlorobenzyl)-1-azoniumbicyclo[2.2.2]octane
[0152] Obtained 131 mg (45%) of a yellow oil. IR (film, cm -1 ): 1694. 1 H-NMR (CDCl 3): 7.60-7.16(m, 14H), 5.03(m, 1H), 4.92(dd, 2H), 4.80(s, 2H), 4.10(m, 1H), 3.77(m, 3H), 3.35(m, 1H), 2.78(m, 1H), 2.28(m, 1H), 1.98(m, 2H), 1.78(m, 1H), 1.60(m, 1H).
Embodiment 3
[0153] Example 3: (R)-3-(Benzylphenylcarbamoyloxy)-1-(5-methylsulfanyl-[1,3,4]thiadiazol-2-ylsulfur chloride Alkylmethyl)-1-azoniumbicyclo[2.2.2]octane
[0154] Obtained 77 mg (53%) of a white solid. 1 H-NMR (CDCl 3 ): 7.27-7.18(m, 10H), 6.97(t, 2H), 6.82(dd, 2H), 5.12(dd, 1H), 4.82(m, 2H), 4.34(s, 2H), 4.30-4.05( m, 3H), 4.05-3.70 (m, 4H), 3.05 (dd, 1H), 2.33 (m, 1H), 2.10-1.50 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com