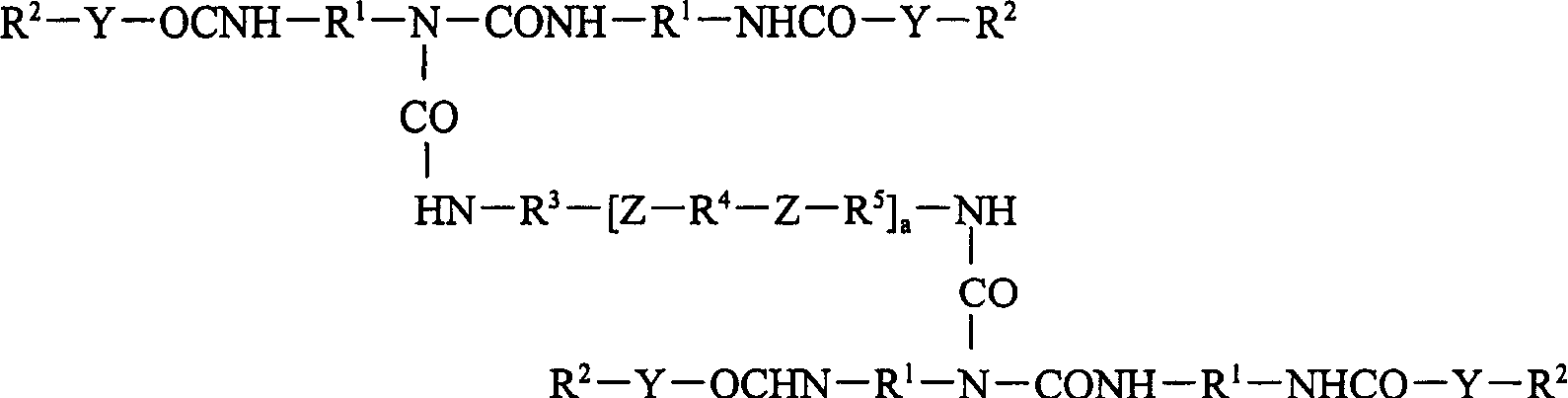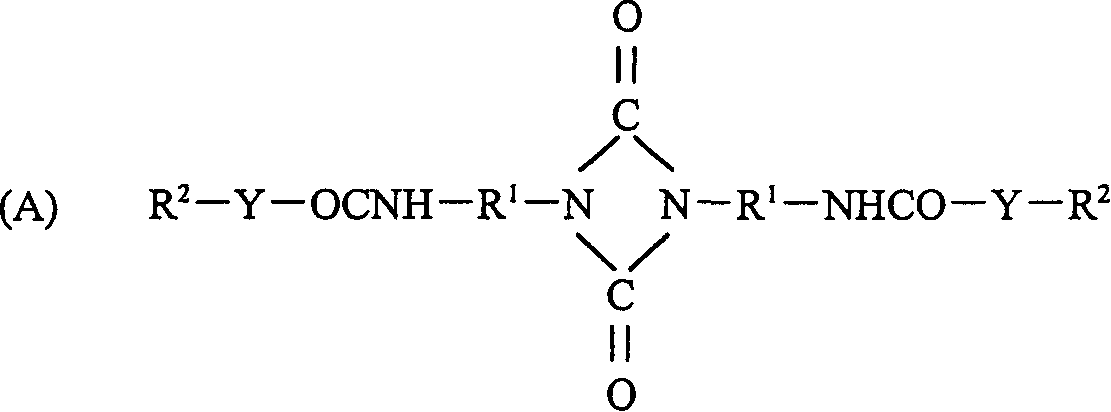Biuret compounds, their preparation and use and intermediates for their preparation
A compound, biuret technology, applied in the field of biuret compound and its preparation, use and intermediates in the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] At room temperature, 79.6 g (0.2 mol) of hexamethylene diisocyanate uretdione (Desmodur N3400 from Bayer) and 300 g (0.4 mol) methoxypolyethylene glycol 750, these initial charges were heated to 80°C. The reaction was allowed to proceed to such an extent that isocyanate was no longer detected. The reaction mixture was then cooled to 50 °C.
[0054] Preparation of component B of the present invention:
Embodiment 2
[0056] At room temperature, 168 g (0.3 mol) of dimer acid, 46.4 g (0.4 mol) of hexamethylenediamine, and 92 g of Shellsol were continuously charged to a 1-liter three-necked flask equipped with a stirrer, reflux condenser, and thermometer. A (high boiling aromatic hydrocarbon solvent, Shell), and these initial charges were heated slowly to 160°C. The water released slowly during the reaction was separated azeotropically by a water separator. When the acid value is less than 3, the reaction ends. The reaction mixture was subsequently cooled to 50°C.
[0057] The preparation of the biuret adduct of the present invention:
Embodiment 3
[0059]At room temperature, 103.6 g (0.1 mol) of the reaction product from Example 1 and 153.1 g (0.05 mol) of the reaction product from Example 2 were continuously charged to a 1-liter three-necked flask with a stirrer, a reflux condenser and a thermometer. reaction product, the mixture was heated to 80°C. The reaction mixture was stirred for an additional 3 hours until the amine number was less than 3. The product was then diluted to 50% solids with isobutanol.
[0060] Example
uretdione
Amine / Alcohol Component
4
Hexamethylene diisocyanate uretdione
5
Hexamethylene diisocyanate uretdione
6
Hexamethylene diisocyanate uretdione
Jeffamine M600
7
Hexamethylene diisocyanate uretdione
Methoxypolyethylene glycol M350
8
Hexamethylene diisocyanate uretdione
Methoxypolyethylene glycol M500
9
Hexamethylene diisocyanate uretdione
Jeffamine M2070 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com