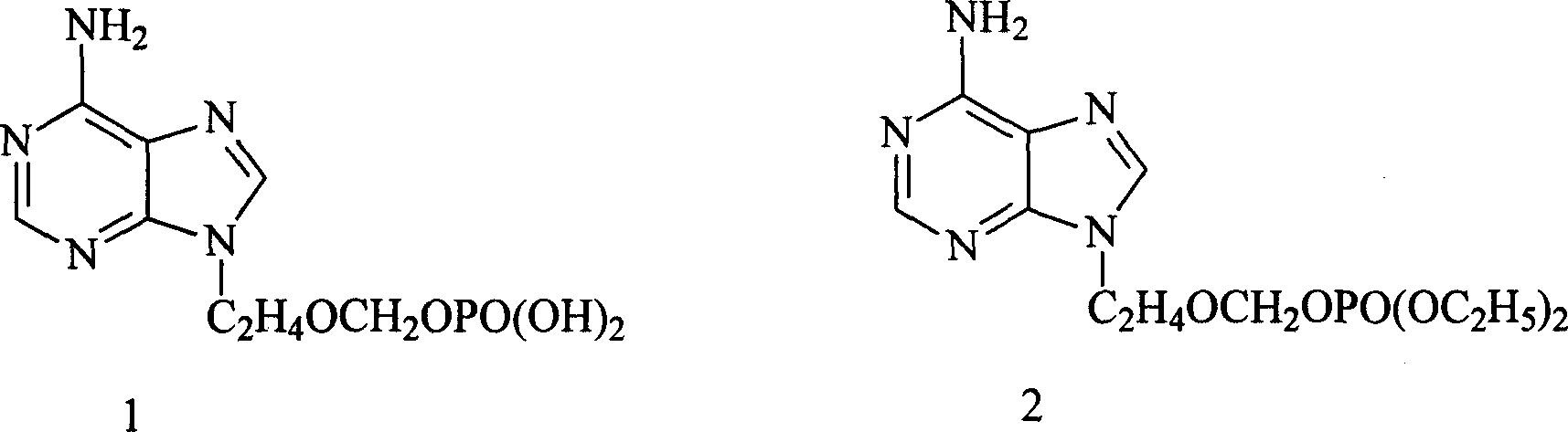
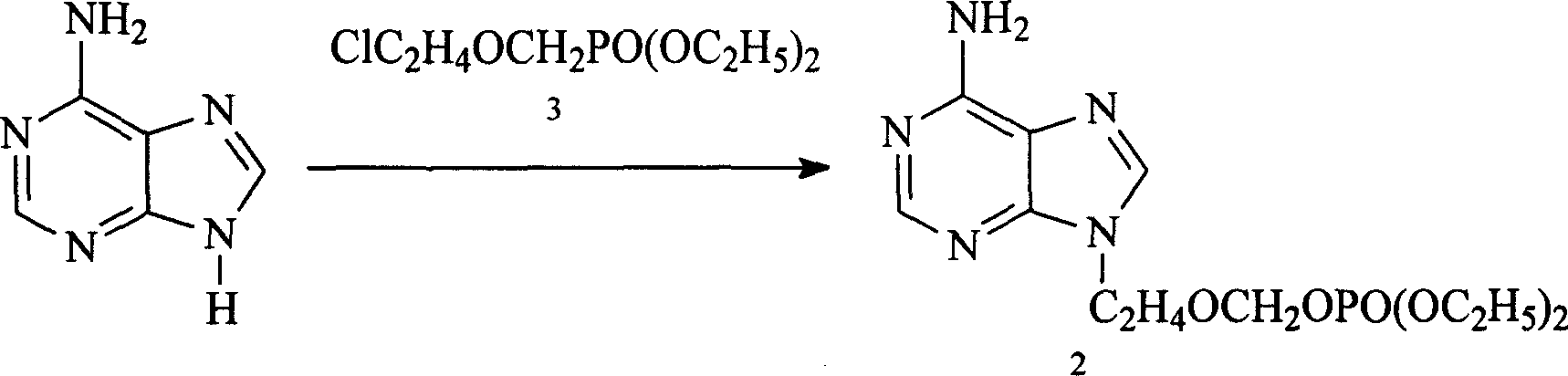
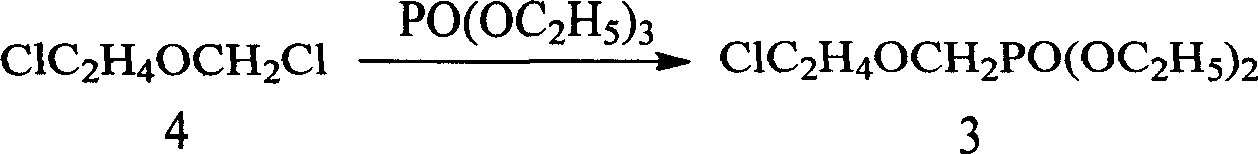Method for preparing 9-(diethoxy phosphoryl methoxy ethyl)-adenine
A technology of diethoxyphosphorylmethoxyethyl and adenine, applied in the field of preparation of 9--adenine, which can solve the problems of high price and rare raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The method for preparing 9-(diethoxyphosphorylmethoxyethyl)-adenine in the present invention comprises the steps of:
[0039] (1) In the presence of anhydrous aluminum trichloride, use paraformaldehyde, 2-chloroethanol, diethyl phosphite and dry hydrogen chloride gas as raw materials, and react at 90 ° C to 125 ° C for 6 to 8 hours , Distilled under reduced pressure, collecting the fraction at 140-150°C / 10mmHg to obtain diethoxyphosphorylmethoxyethyl chloride (compound 3);
[0040] Wherein: the recommended consumption of anhydrous aluminum trichloride is that its mol ratio with paraformaldehyde is 1: 3~4; The preferred mol ratio of paraformaldehyde, 2-chloroethanol and diethyl phosphite is 1: (0.5 ~1.1): (0.5 ~ 1.5), the best is 1: (0.8 ~ 1.0): (0.7 ~ 1.0); the preferred molar ratio of diethoxyphosphorylmethoxyethyl chloride and adenine is 1: (0.5 ~ 1.1), the best is 1: (0.8 ~ 1.0).
[0041] (2) The diethoxyphosphorylmethoxyethyl chloride and adenine produced by step ...
Embodiment 1
[0045] Paraformaldehyde (chemically pure, Shanghai Experimental Reagent Co., Ltd.) (94g, 3.24mol), 2-chloroethanol (255g, 3.16mol), anhydrous aluminum trichloride (139g, 1.04mol) and diethyl phosphite ( Industrial product, Yixing Manqiu Chemical Co., Ltd.) (385g, 2.5mol), at 25°C, feed dry hydrogen chloride gas (generated by concentrated sulfuric acid and concentrated hydrochloric acid) into the system under stirring, then pass through concentrated sulfuric acid and anhydrous Sodium sulfate drying), when all the solids in the mixture are dissolved, stop feeding hydrogen chloride, heat up to 90°C within 1 hour, react for 4 hours, then heat up to 125°C for 2 hours, then change the reaction device to vacuum distillation device, collect 140~150 DEG C / 10mmHg cuts, obtain yellowish liquid (250g, 1.04mol), purity 96% (gas chromatography content), yield 41.6% (based on diethyl phosphite).
[0046]Under stirring, in 2000mL DMF (900mL), add adenine (25g, 0.185mol), anhydrous potassium c...
Embodiment 2
[0048] In a 1000L glass-lined reactor, add paraformaldehyde (47kg, 1.62kmol), 2-chloroethanol (127.5kg, 1.58kmol), anhydrous aluminum trichloride (69.5kg, 520mol) and diethyl phosphite (192.5 kg, 1.25kmol), at 25°C, feed dry hydrogen chloride gas into the system under stirring (generated by concentrated sulfuric acid and concentrated hydrochloric acid, and then dried by concentrated sulfuric acid and anhydrous sodium sulfate), when all the solids in the mixture dissolve Afterwards, stop feeding hydrogen chloride, heat up to 90°C within 1 hour, react for 4 hours, then heat up to 125°C for 2 hours, then switch the reaction device to a vacuum distillation state, collect 140-150°C / 10mmHg fractions, A slightly yellow liquid (125kg, 520mol) was obtained with a purity of 96% (gas chromatography content) and a yield of 41.6% (based on diethyl phosphite).
[0049] Add DMF (450L) to a 1000L glass-lined reactor, add adenine (12.5kg, 92.5mol) and anhydrous potassium carbonate (25kg, 180mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com