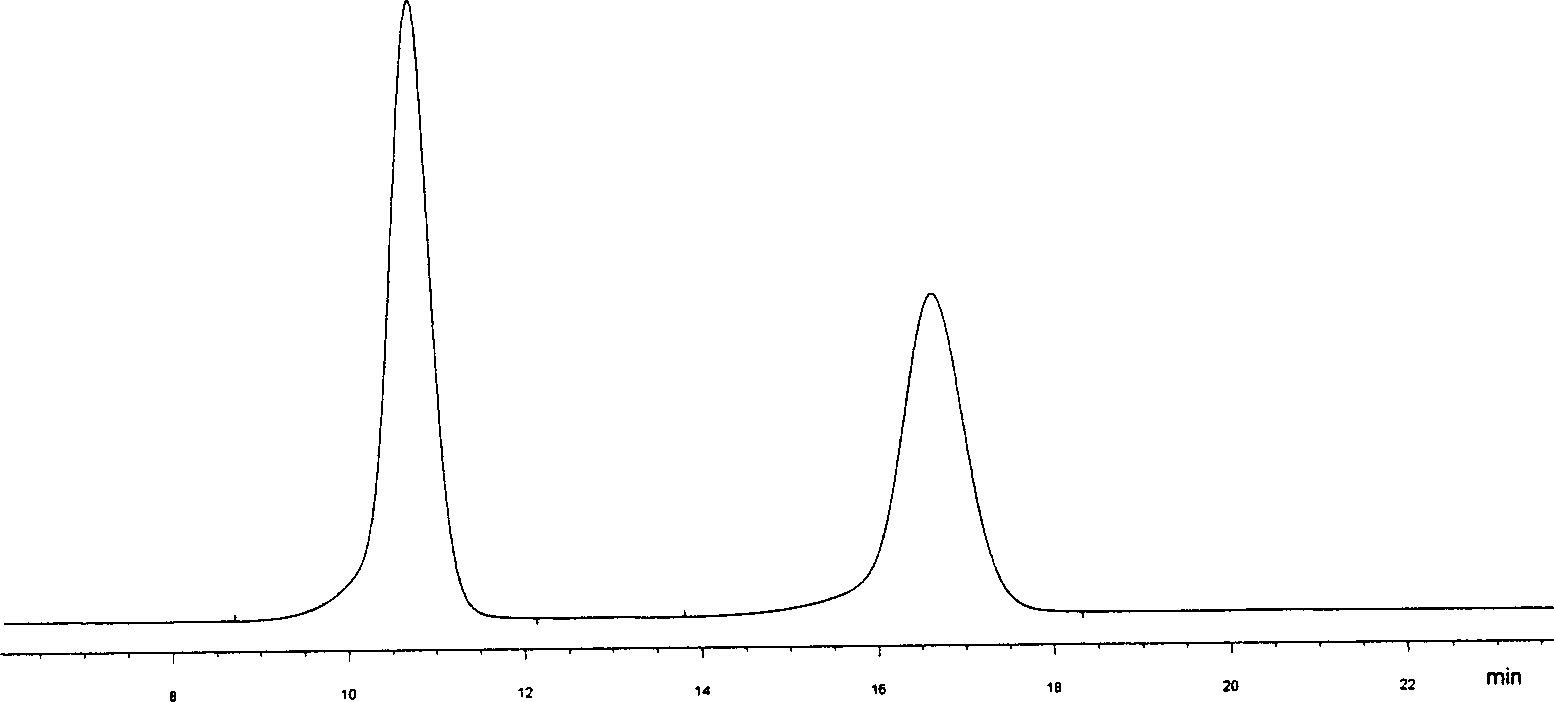
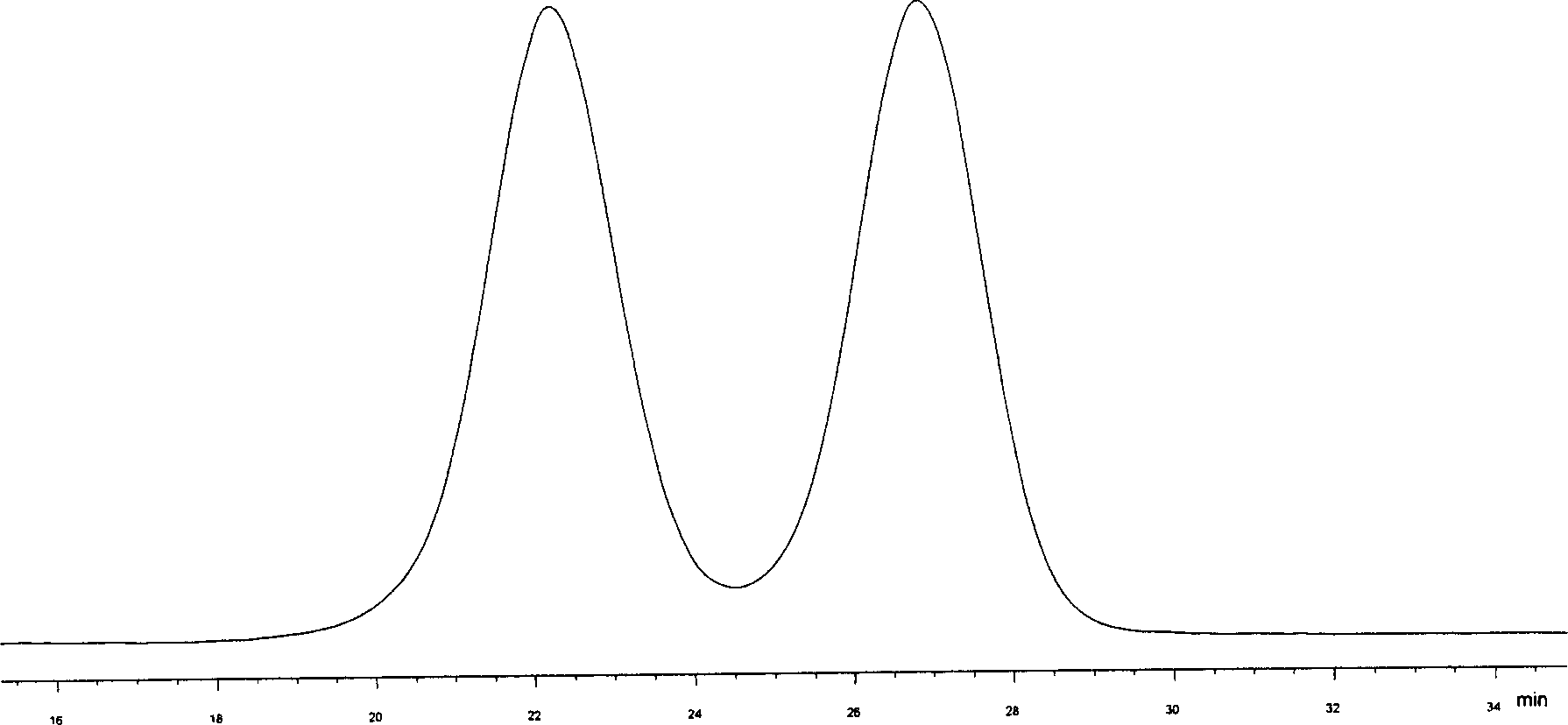
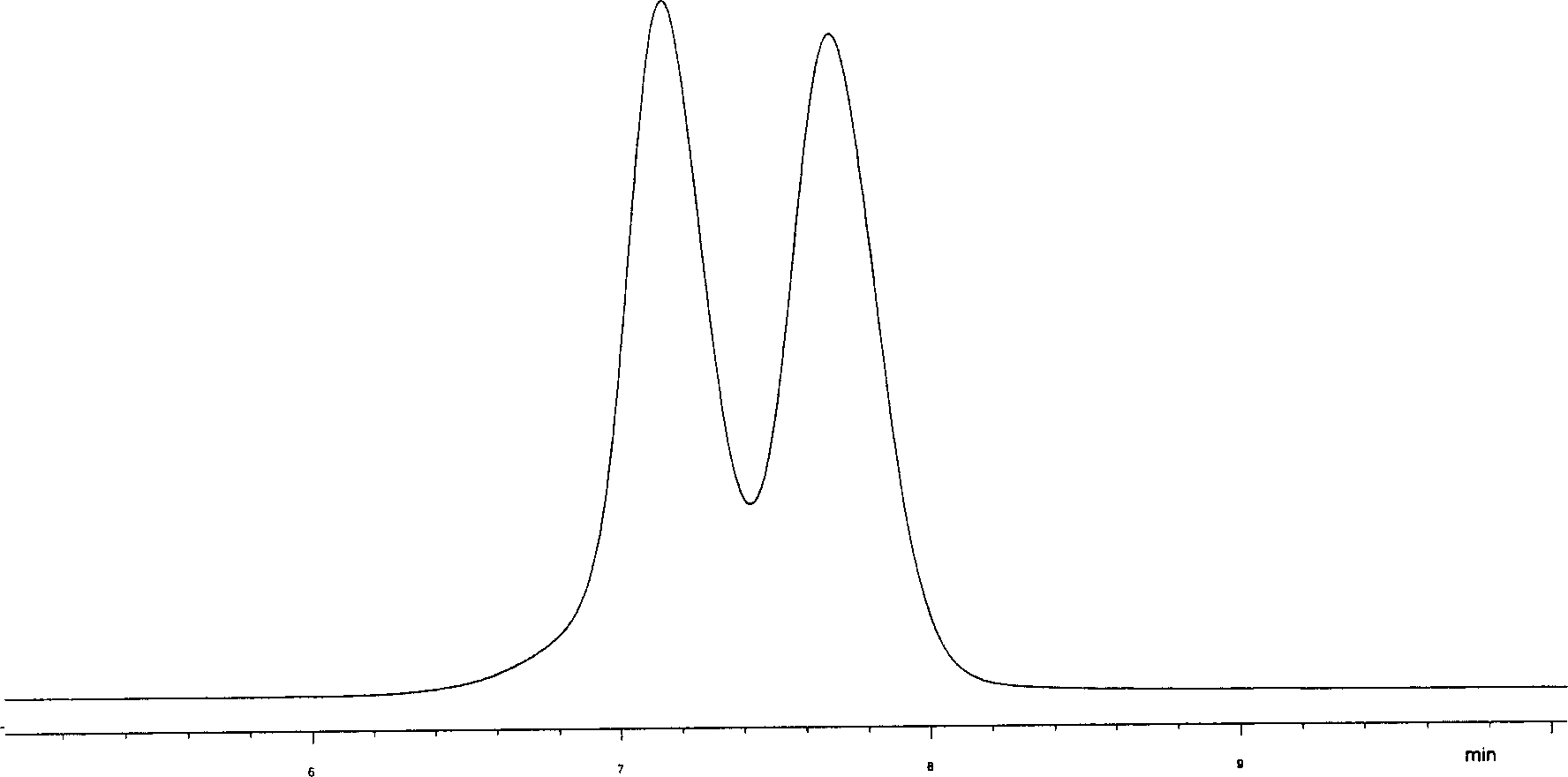
Biological micromolecule or its analogue bonded chiral stationery phase
A chiral stationary phase and bonding technology, applied in other chemical processes, chemical instruments and methods, etc., can solve problems such as lack of stability of bidentate structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A double-tooth structure connecting arm silica gel. The preparation method of chiral chromatographic stationary phase has two kinds, and a kind of is that linking arm and chiral selector react with silane coupling agent earlier, then couple on silica gel (William H.Pirkle, J.Chromatogr.A, 1992 , 589, 45). The other is to couple the silane coupling agent to the silica gel first, and then react with the connecting arm or the chiral selector to obtain the stationary phase (US Patent Publication No. US2004 / 0226889 A1). The latter preparation method is preferred in the present invention. Silica gel is coupled with a silylating reagent. To 4.0 g of pretreated silica gel (Kromasil, 5 μm, 100 Å, Switzerland), 120 mL of dry toluene and 30 mL of bis(trimethoxysilylpropyl)amine (Gelest, Inc., Morrisville, PA.USA) were added. Under nitrogen protection, heat in an oil bath at 140°C for 24 hours. After cooling, filter, wash the solid with toluene and acetone, and dry it in vacuum ...
Embodiment 2
[0066]In 20 mL of anhydrous DMF, add 3.8 grams of bonded silica gel in Example 1, 4 grams of HATU (Sigma-Aldrich Corp. St. Louis, MO, USA) and 1.4 grams of DIPEA (Sigma-Aldrich Corp. St. Louis, MO , USA), and then added 4.0 g of fluorenylmethoxycarbonyl-protected proline (Fmoc-Pro-OH) (CHEM-IMPEX INTERNATIONAL, IL, USA). After stirring for 12 hours, it was filtered and the silica gel was washed with DMF, methanol and dichloromethane. The Fmoc protecting group was reacted with 20 mL of piperidine in 20% DMF for 60 minutes to remove it. Filter and wash the solid with DMF and dichloromethane. dry. This is the first coupling process. After the obtained silica gel was subjected to the same coupling twice, the obtained silica gel was added to 20 mL of dichloromethane, and then 1.6 g of DIPEA and 1.4 g of trimethylacetyl chloride (Sigma-Aldrich Corp. St. Louis, MO, USA) were added. After stirring for 12 hours, it was filtered and the solid was washed with DMF, methanol and dichlo...
Embodiment 3
[0072] According to the method of Example 1, 3-aminopropyl silica gel was prepared. For the synthesis of amino-bonded silica for longer chain monodentate linkers, in anhydrous DMF, add 4.0 g of this bonded silica, 750 mg of DIC (Sigma-Aldrich Corp. St.Louis, MO, USA), 800 mg HOBT (Sigma-AldrichCorp.St.Louis, MO, USA) and 700 mg of 4-(1-Boc piperidine) acetic acid (Sigma-AldrichCorp.St.Louis, MO, USA), stirred for 12 hours, filtered, and used for silica gel DMF, methanol and dichloromethane washes. The Boc protecting group was removed with 50% trifluoroacetic acid in dichloromethane for 60 minutes, and the solid was treated with DMF, dichloromethane and 50% N,N-diisopropylethylamine (DIPEA) (Sigma-Aldrich Corp.St .Louis, MO, USA) dichloromethane solution washes, and dries, obtains the amino-bonded silica gel of the monodentate connecting arm of long chain. According to the method of Example 2, using Fmoc-Pro-OH, after three times of coupling and end-capping, the stationary ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com