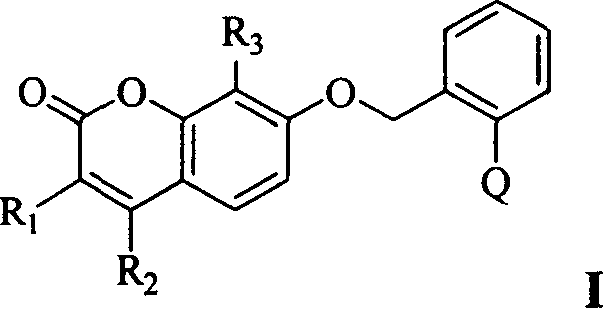
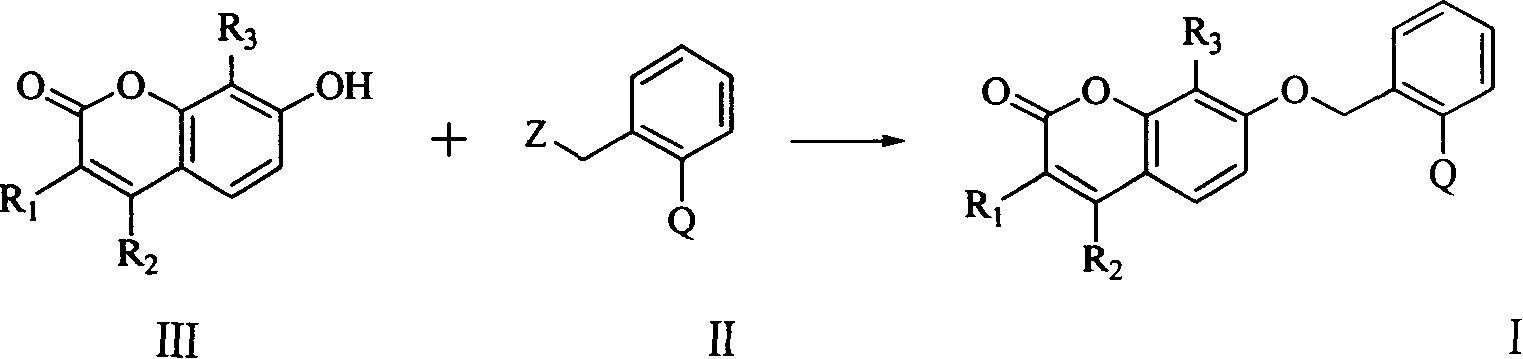
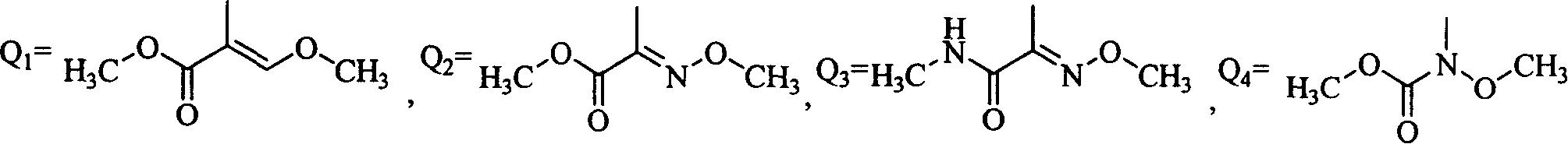Coumarin kind compound and its preparation and application
A technology of coumarins and compounds, applied in the fields of fungicides and agricultural insecticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0070] Example 1 Preparation of Compound 5
[0071]
[0072] At room temperature, 0.2 g of 60% sodium hydride was added to the reaction flask, washed with petroleum ether, 30 ml of dry N,N-dimethylformamide was added to it, stirred for half an hour, and 0.5 g of compound (III -1), continue to stir until no gas is released, add 0.8 g of compound (II-1), and continue to stir for 3 hours. The reaction mixture was poured into ice water, extracted with ethyl acetate 3 times, the extracts were combined, washed 3 times with saturated brine, dried, filtered, and concentrated under reduced pressure to obtain 5 g of oily liquid. Column chromatography obtained 0.68 g of the title compound as a pale yellow solid product with a yield of 68%. Melting point: 131-133°C.
[0073] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0074] δppm 2.18 (3H, s), 2.36 (3H, s), 3.77 (3H, s), 3.83 (3H, s), 5.17 (2H, s), 6.84-6.85 (1H, d), 6.89-6.92 (1H) , M), 7.39 (3H, m), 7.4...
example 2
[0075] Example 2 Preparation of compound 49
[0076]
[0077] At room temperature, a mixed solution containing 0.85 g of anhydrous potassium carbonate, 0.6 g of compound (III-2), 0.93 g of compound (II-1) in 20 ml of methyl ethyl ketone was heated under reflux and stirred for reaction for 5 hours, and the reaction mixture was poured into Extract 3 times with ethyl acetate in ice water, combine the extracts, wash 3 times with saturated brine, dry, filter, and concentrate under reduced pressure to obtain a yellow solid as a crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:2) was used to obtain 0.89 g of the title compound as a solid, with a yield of 75%. Melting point: 83-85°C.
[0078] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0079] δppm 2.37 (3H, s), 3.77 (3H, s), 3.84 (3H, s), 5.18 (2H, s), 6.88-6.89 (1H, d), 7.39 (1H, m), 7.41 (3H, m ), 7.45 (1H, m), 7.48 (1H, m).
example 3
[0080] Example 3 Preparation of Compound 106
[0081]
[0082] At room temperature, a mixture containing 0.3 g of anhydrous potassium carbonate, 0.22 g of compound (III-3), 0.30 g of compound (II-2) in 10 ml of methyl ethyl ketone was heated under reflux and stirred for reaction for 5 hours, and the reaction mixture was poured into Extract 3 times with ethyl acetate in ice water, combine the extracts, wash 3 times with saturated brine, dry, filter, and concentrate under reduced pressure to obtain a yellow solid as a crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:2) yielded 0.18 g of the title compound as a solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com