Method for producing recombined human proinsulin
A technology of human insulin and original protein, applied in the field of genetic engineering, which can solve the problems of low yield of active products and increased post-processing complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
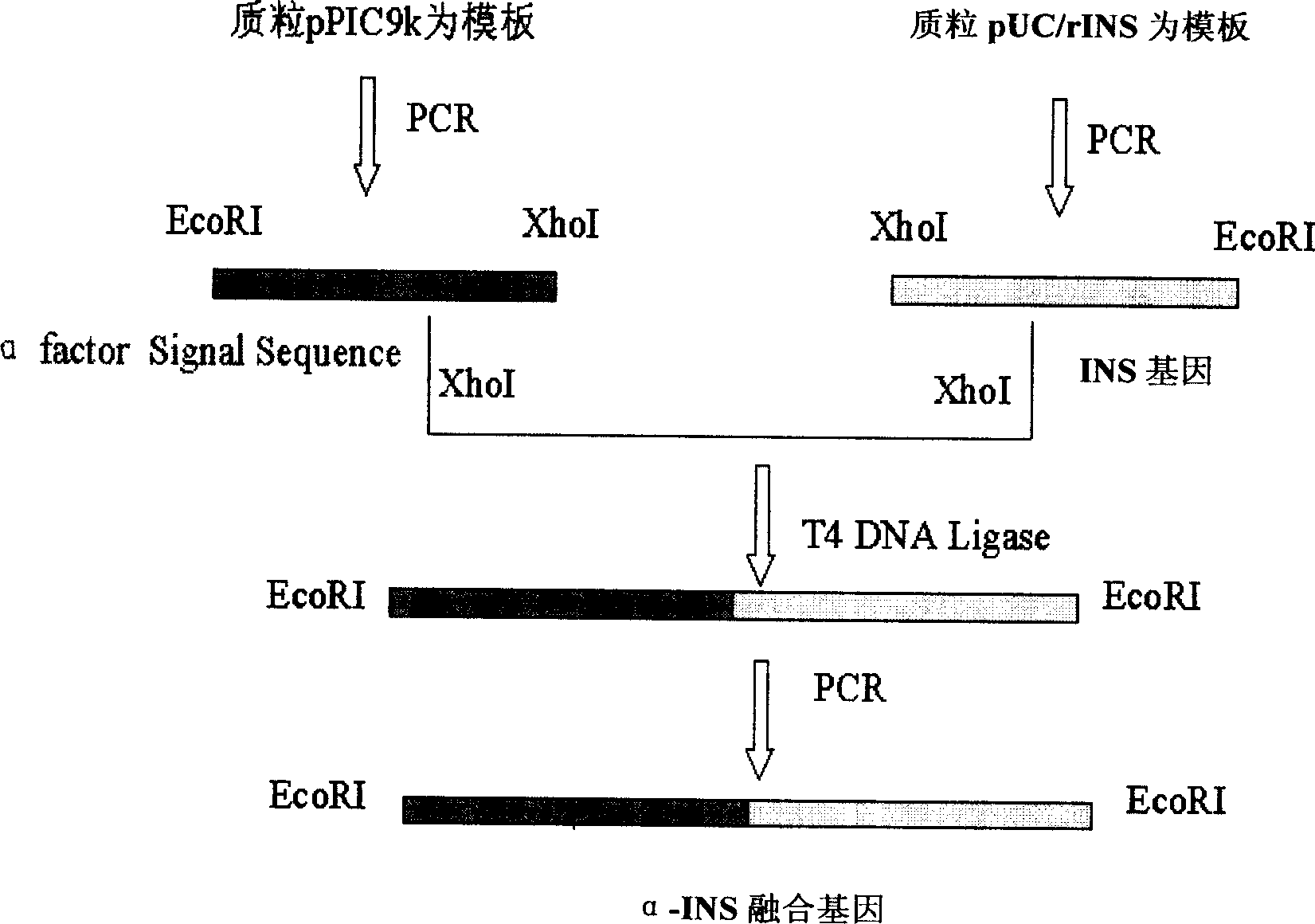
[0052] The acquisition of embodiment 1 fusion gene and the construction of expression plasmid
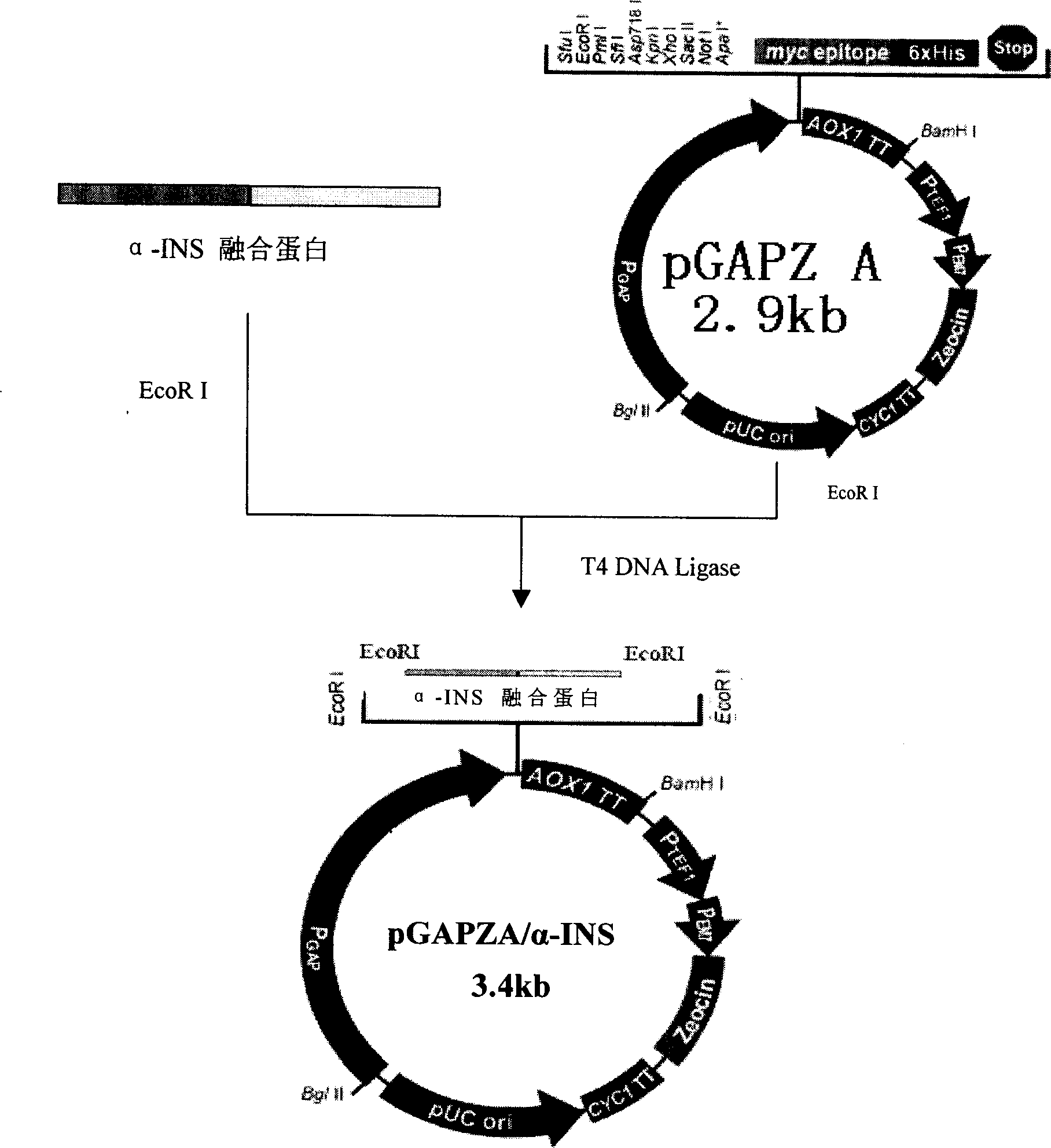
[0053] The gene sequence of the human proinsulin molecule (INS) of the novel N-terminal leader sequence was synthesized from the whole gene, the gene was cloned into pUC19 and sequenced and verified, and then the fusion sequence of the fusion protein α-INS was constructed in vitro by molecular biological methods ( See Figure 1), using the plasmid pUC19 / rINS containing the rINS coding sequence as a template, the INS gene that can be fused with the Saccharomyces cerevisiae α-factor leader peptide sequence in the correct frame was obtained by PCR amplification. At the same time, using the plasmid pPIC9K (purchased from Invitrogen) containing the Saccharomyces cerevisiae α-factor leader peptide coding sequence as a template, the α-factor leader peptide sequence was amplified by PCR. The purified above two PCR products were respectively digested with restriction enzyme XhoI, and the two ...
Embodiment 2
[0055] Example 2 Screening of Highly Expressed Recombinant Human Proinsulin Engineering Strain
[0056] The constructed recombinant plasmid pGAPZA / α-INS was prepared in large quantities, linearized, electroporated and transformed into host cell P. pastoris GS115, coated with YPDS plates containing different concentrations of the antibiotic Zeocin to screen high-resistance positive clones, and a small shaker expression experiment was performed The engineered yeast strain (containing α-INS) with high expression of recombinant human proinsulin was screened.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com