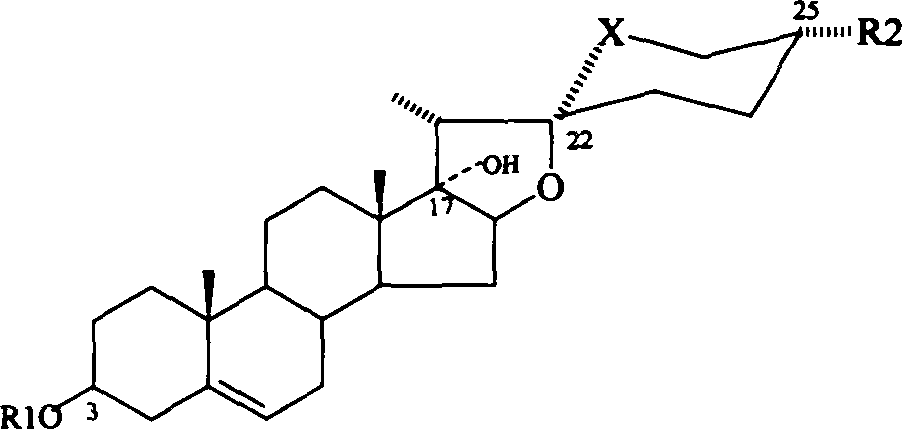
Solid-state molecular dispersible preparation of pennogenin compound
A technology of pinanoside and molecular dispersion, which is applied in the field of special preparations, can solve the problems of low bioavailability, and achieve the effects of overcoming slow dissolution, improving bioavailability, and easy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] result:
[0047] (1), in the above examples, the dissolution rate and bioavailability of pinanosides were obviously improved. The preparation with cyclodextrin as the main auxiliary material has excellent stability and is not easy to age.
[0048] (2) Water-soluble polymers such as PVP and PEG obviously improve the dissolution rate of the pinanoside compound-cyclodextrin complex, and obviously increase the bioavailability of the pinanoside compound.
[0049] (3) The hydroxy acid can obviously improve the dissolution rate of the pinanoside compound-cyclodextrin complex, and obviously improve the bioavailability of the pinanoside compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com