Pregabalin intermediate and process for preparing same
A technology of isobutylglutarimide and cyclization reaction, which is applied in the field of pregabalin intermediates and its preparation, can solve the problems of long synthetic route, difficult separation, high toxicity of solvent-chloroform, etc., and achieve cost-effective, Mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
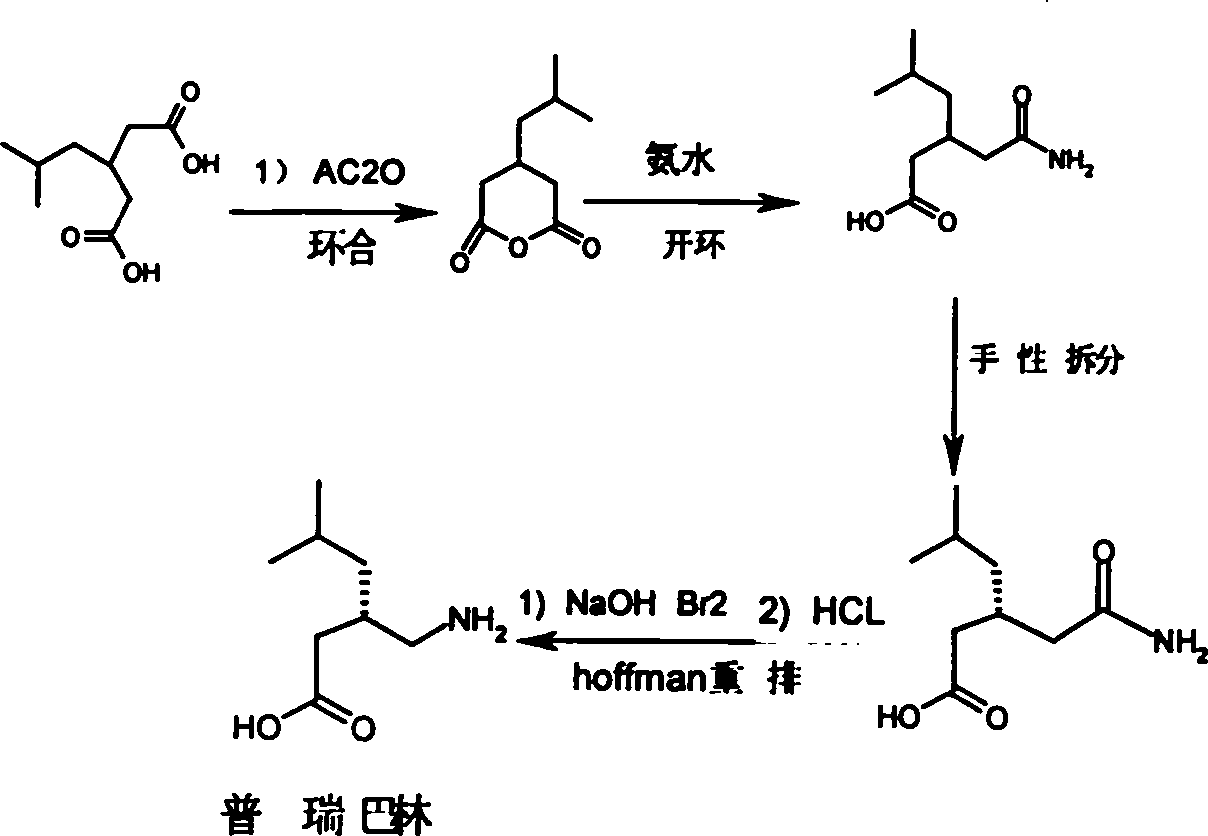
[0034] 1) Preparation of 3-isobutylglutaric acid (refer to Organic Process Research & Development 1997, 1, 26-38)
[0035]Ethyl cyanoacetate (187.2g, 1.65mol), isovaleraldehyde (156.3g, 1.81mol) and 210ml of n-hexane, n-dipropylamine (1.65g, 1.62mmol) were placed in a reaction flask, refluxed to separate water until no water came out, The solvent was concentrated under reduced pressure to dryness, diethylphthalate (317.1g, 1.98mol) and n-dipropylamine (16.8g, 165mmol) were added, stirred at 50°C for 1 hour, then poured into 900ml of 6N hydrochloric acid, and refluxed for 72 hours. Cool the toluene for extraction, and concentrate the extract to dryness to obtain 3-isobutylglutaric acid (261.9g, yield 85%). NMR 1 HNMR and infrared IR (KBr) are consistent with the literature.
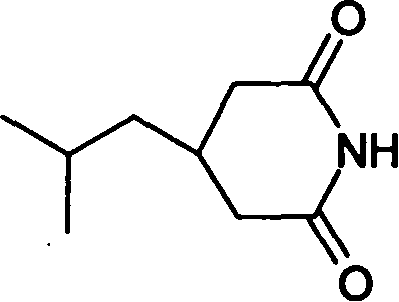
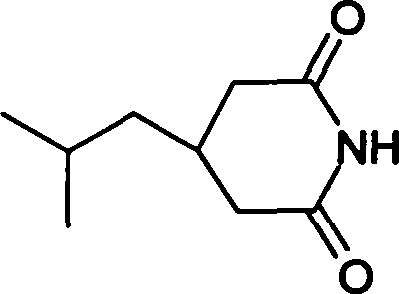
[0036] 2) Preparation of 3-isobutylglutarimide
[0037]
[0038] Add 200.0g (1.06mol) of 3-isobutylglutaric acid and 63.8g (1.06mol) of urea into a 1000ml three-necked flask. Heat the oil bath to 160...
Embodiment 2
[0050] 1) Preparation of 3-isobutylglutaric acid
[0051] Same as step 1) in Example 1
[0052] 2) Preparation of 3-isobutylglutarimide
[0053] Add 200.0g (1.06mol) of 3-isobutylglutaric acid and 127.7g (2.12mol) of urea into a 1000ml three-necked flask. Heat the oil bath to 150°C, react at 150-170°C for 2 hours, then cool to 80°C, add 200ml of water, 400ml of ethanol and 15g of activated carbon, heat and reflux for 30 minutes, filter while it is hot, cool the filtrate, filter, and vacuum at 50°C After drying, 166 g (yield: 92.3%) of 3-isobutylglutarimide can be obtained as white flaky crystals with a melting point of 137-138°C.
[0054] Mass spectrum MS: 125.9, 168.0, 168.9
[0055] Nuclear magnetic resonance 1HNMR (CDCl3): δ: 8.01 (s, 1H, exchangeable with D2O), 2.72-2.68 (d, 2H), 2.24-2.21 (d, 2H), 1.67-1.62 (m, 2H), 1.28-1.24 (t, 2H), 0.92 (s, 6H)
[0056] Infrared IR (KBr): 3198, 3088, 2955, 1735, 1686, 1422, 1384, 1291, 1269cm-1
[0057] 3) Preparation of 3-isobut...
Embodiment 3
[0062] 1) Preparation of 3-isobutylglutaric acid
[0063] With embodiment one.In step 1)
[0064] 2) Preparation of 3-isobutylglutarimide
[0065] Add 200.0 g (1.06 mol) of 3-isobutylglutaric acid and 220 g (3.18 mol) of 25% ammonia water into a 1000 ml three-necked flask. (During the experiment, due to excessive ammonia water, although there was volatilization, it did not affect the reaction) After concentrating the ammonia water to dryness, heat the oil bath to 100°C, react for 2 hours, then cool to 80°C, add 400ml of water, 200ml of ethanol and activated carbon 15g, reheated and refluxed for 30 minutes, filtered while hot, cooled the filtrate, filtered, and dried in vacuum at 50°C to obtain 164g of 3-isobutylglutarimide (yield 91%), white flaky crystals, melting point 137- 138°C.
[0066] Mass spectrum MS: 125.9, 168.0, 168.9
[0067] Nuclear magnetic resonance 1HNMR (CDCl3): δ: 8.01 (s, 1H, exchangeable with D2O), 2.72-2.68 (d, 2H), 2.24-2.21 (d, 2H), 1.67-1.62 (m, 2H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com