Slow release medicinal composition for treating anxiety and its preparing method
A slow-release drug and composition technology, applied in the field of medicine, can solve the problems of poor patient taking compliance and large fluctuation of blood drug concentration, and achieve the effects of avoiding peak-to-valley phenomenon, stabilizing blood drug concentration and improving compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
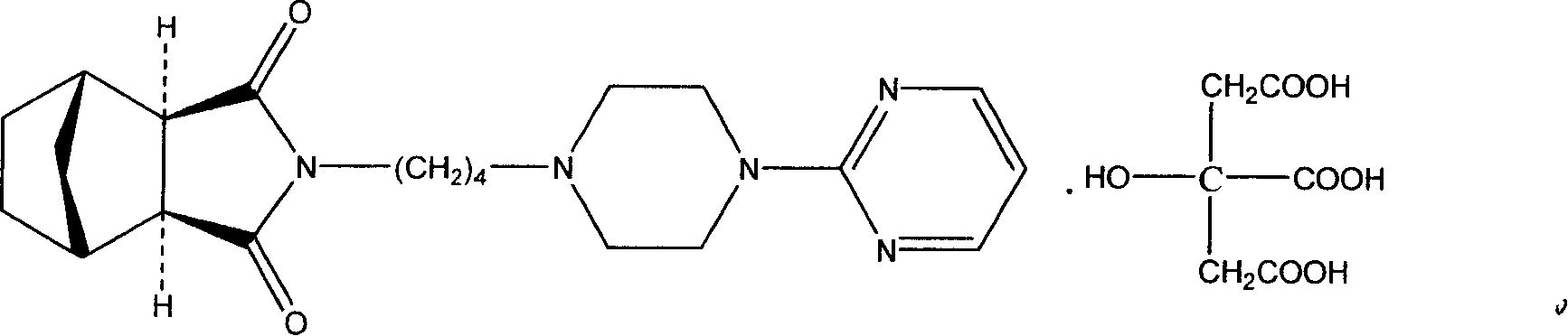
[0041] Tandospirone Citrate 16.2g
[0042] Hydroxypropylmethylcellulose (HPMC K4M) 64.8g
[0043] 75% ethanol 12ml
[0044] Lactose 110g
[0047] Preparation process: Mix tandospirone citrate raw material with hydroxypropyl methylcellulose and lactose evenly, sieve, add 75% alcohol solution to make soft material, pass through 20-mesh sieve to make wet granules, and store at 50°C Dry left and right, sieve with 20 meshes, add magnesium stearate and talcum powder, mix evenly and press into tablets. The weight of the tablet is 0.2g, and a total of 1000 tablets are made.
[0048] Animal experiment: Take 12 New Zealand rabbits, half male and half female, weighing (4.5-5) kg, and randomly divide them into 2 groups, fast for 18 hours before administration, and administer with 10 mg tandospirone citrate / kg. Blood was taken from rabbit ear vein, and the pharmacokinetic parameters were measured. The results showed that compared...
Embodiment 2
[0053] Tandospirone Citrate 30g
[0054] Hydroxypropylmethylcellulose (HPMC K15M) 50g
[0055] Methylcellulose 50g
[0056] 75% ethanol 13ml
[0057] Lactose 70g
[0060] Preparation process: Mix tandospirone citrate raw material with hydroxypropyl methylcellulose, methylcellulose, lactose, and evenly, sieve, add 75% ethanol solution to make soft material, pass through 20-mesh sieve to make wet Granules, dried at about 50°C, sieved with 20 meshes, added magnesium stearate and talcum powder, mixed evenly and pressed into tablets. The weight of the tablet is 0.2g, and a total of 1000 tablets are made.
[0061] The animal experiment method is as example 1, and the results are shown in Table 2
[0062] t peak (h)
C max (ng / ml)
AUC(ng.h / ml)
Sustained Release Tablets
Ordinary film
3.4±3.1 *
0.8±0.5
1.7±0.8 *
3.5±1.3
44.7±12.3 *
13.1±3.5
[0063] ...
Embodiment 3
[0066] Tandospirone Citrate 10g
[0067] Sodium Alginate 25g
[0068] Methylcellulose 50g
[0069] Carboxymethylcellulose 25g
[0070] 75% ethanol solution 10ml
[0071] Cornstarch 100g
[0072] Preparation process: Mix tandospirone citrate raw material with sodium alginate, methyl cellulose, carboxymethyl cellulose, and corn starch evenly, sieve, add 75% ethanol solution to make soft material, and pass through a 20-mesh sieve Prepare wet granules and compress into tablets. The weight of the tablet is 0.2g, and a total of 1000 tablets are made.
[0073] The animal experiment method is as example 1, and the results are shown in Table 3
[0074] t peak (h)
C max (ng / ml)
AUC(ng.h / ml)
Sustained Release Tablets
Ordinary film
3.9±1.4 *
1.1±1.2
1.4±0.3 *
3.4±2.1
43.2±10.3 *
11.4±6.5
[0075] * Compared with ordinary tablets, P<0.05
[0076] Side effects: No side effects occurred.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com