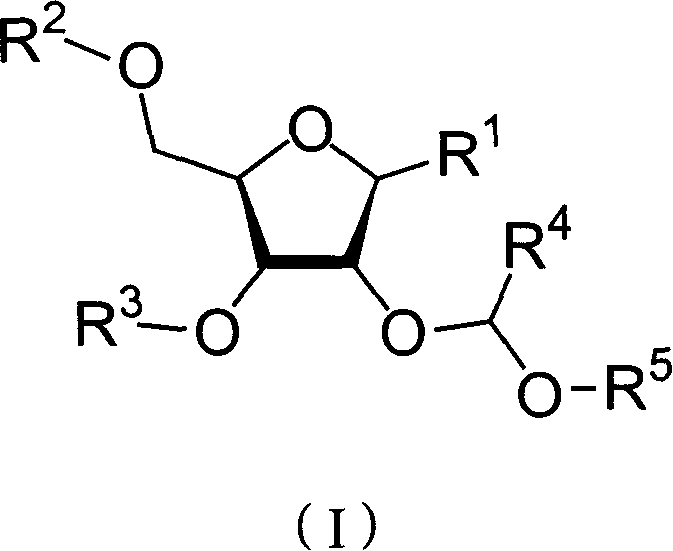
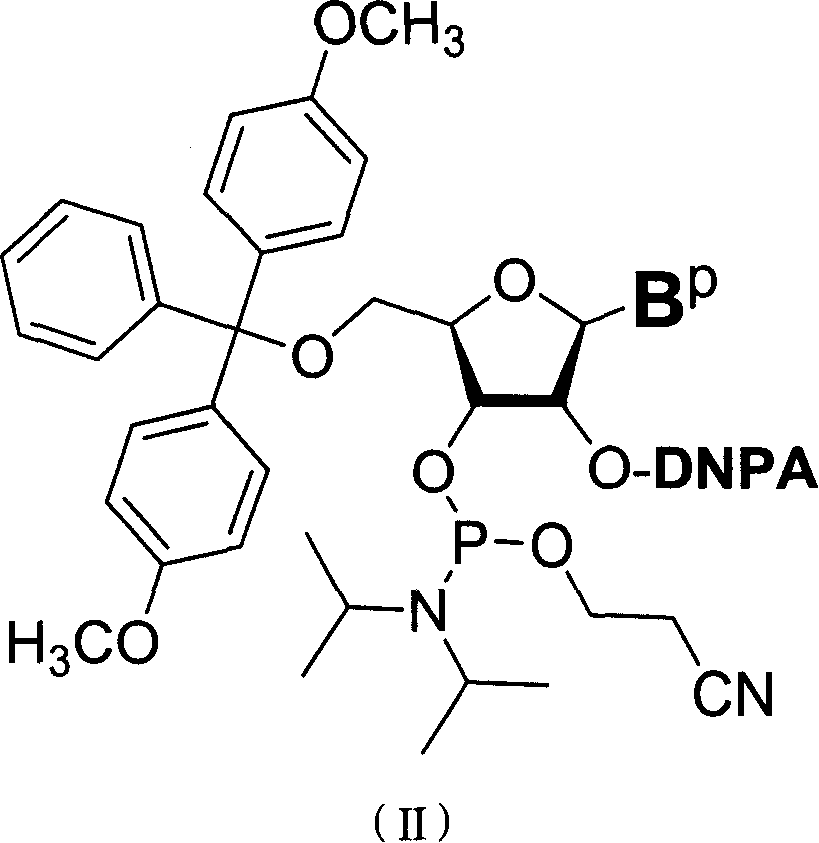
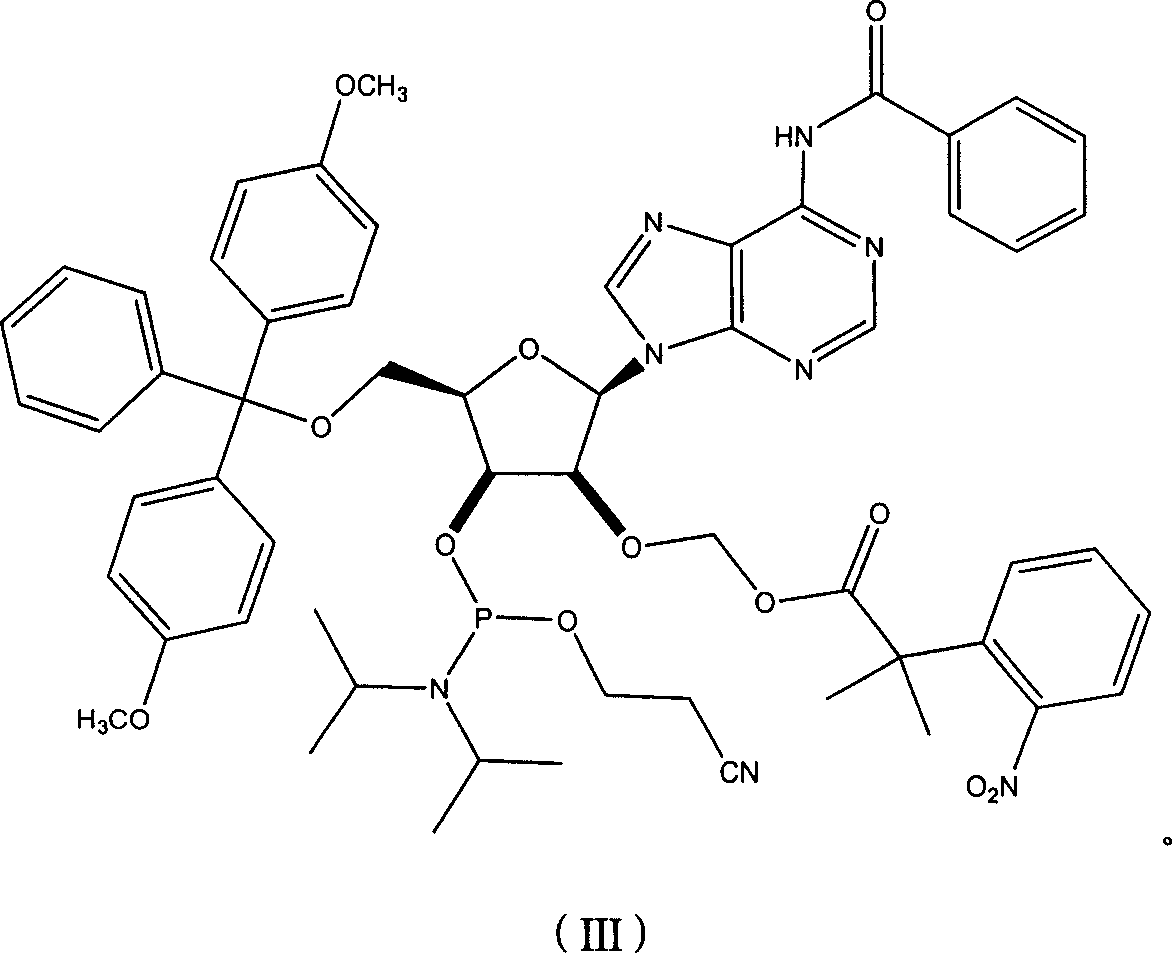
Nucleoside phosphoramidite used in RNA oligo-nucleotide synthesis and its synthesizing method
A nucleoside phosphoramidite and nucleoside technology, which is applied in the field of chemical synthesis of oligonucleotides, can solve problems such as poor compatibility, and achieve the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthesis of embodiment 1 adenosine phosphoramidite
[0048] Synthesis of 6-N-benzoyladenosine (compound 1): adenosine (10.68g, 39.96mmol) was dissolved in 200mL pyridine to form a suspension, trimethylchlorosilane (38.1mL, 300mmol) was added therein, at room temperature Under the protection of argon flow, the mixture was fully mixed and reacted for 45 min, then benzoyl chloride (23.1 mL, 200 mmol) was added and mixed for 4 h, the mixture was cooled in an ice bath and 40 mL of water was added, and after 20 min, 29% ammonium hydroxide was slowly added. Aqueous solution (96mL, 713.3mmol), mixed at room temperature for 45min, centrifuged and vacuumed to dry powder, added 320mL of water to dissolve. The solution was washed once with ethyl acetate (100 mL) and crystals formed immediately. After filtration, it was washed successively with 30 mL EtOAc, 40 mL ice water, and 40 mL acetone (twice) to obtain a white solid 6-N-benzoyladenosine (Compound 1) (14.17 g...
Embodiment 2
[0053] The synthesis of embodiment 2 cytidine phosphoramidites
[0054] Synthesis of 4-N-benzoylcytidine (Compound 7): Cytidine (9.72 g, 39.96 mmol) was added to 200 mL of pyrimidine to form a suspension, and then trimethylchlorosilane (38.1 mL, 300 mmol) was added thereto. Mix well at room temperature under the protection of argon flow for 1 h, then add benzoyl chloride (23.0 mL, 200 mmol) at room temperature and mix for 4 h, add 40 mL of water after the mixture is cooled in an ice bath, and add 29% ammonium hydroxide after 20 min Solution (60mL, 445.8mmol), mixed at room temperature for 25min, centrifuged and evacuated to dry powder, added 400mL of water to dissolve. Crystallization occurred after adding 150 mL of EtOAc. After filtration, it was washed successively with 30 mL of ethyl acetate, 30 mL of ice water, and 30 mL of acetone (twice) to obtain 4-N-benzoylcytidine (Compound 7) (13.1 g, 94.4%) as a white solid. R f =0.55 (20% methanol-ethyl acetyl ester)...
Embodiment 3
[0057] The synthesis of embodiment 3 guanosine phosphoramidites
[0058] 6-N-benzoyl-2'-O-DNPA-5'-O-(4,4'-dimethoxytrityl)guanosine-3 was prepared according to the corresponding method in Example 1 '-O-(2-ethylcyano-N,N-diisopropylphosphoramidite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com